
Pharmaceutical Cleaning Validation Market Share, Size, Trends, Industry Analysis Report
By Product (Small Molecule Drug, Peptides, Proteins, Cleaning Detergent); By Test; By Region; Segment Forecast, 2021 - 2028
- Published Date:Oct-2021
- Pages: 113
- Format: PDF
- Report ID: PM2120
- Base Year: 2020
- Historical Data: 2016 - 2019
Report Outlook
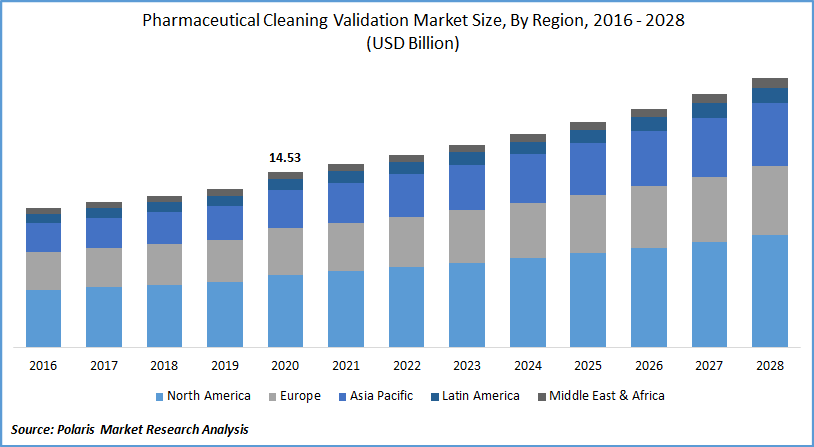
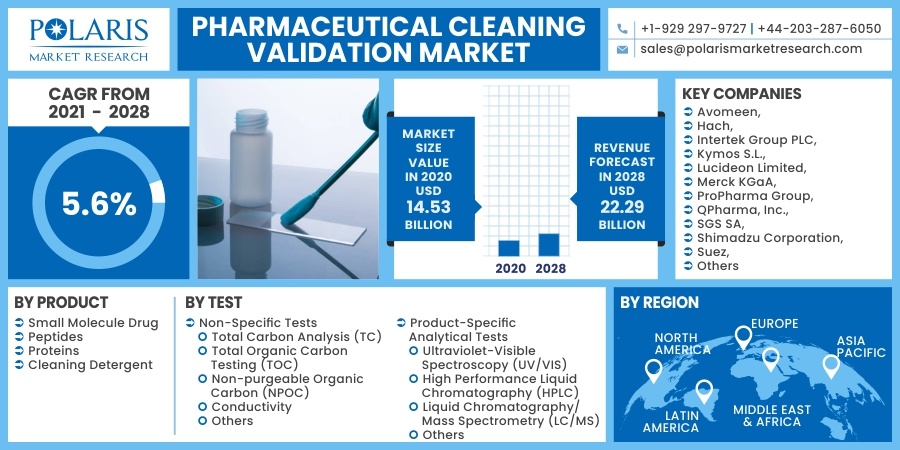
The global pharmaceutical cleaning validation market was valued at USD 14.53 billion in 2020 and is expected to grow at a CAGR of 5.6% during the forecast period. The rising demand for cleaning validation owing to the growth of the pharmaceutical industry is stimulating market growth across the globe. In addition, the rising responsiveness regarding the cleaning validation along with stringent standards implemented by the regulatory bodies may be attributed to the pharmaceutical cleaning validation market demand.
 Know more about this report: request for sample pages
Know more about this report: request for sample pages
Moreover, increasing expenditures on the R&D activities and increasing investment and spending by the chief competitors across the market are further factors that accelerate the pharmaceutical cleaning validation industry development in the approaching years.
The outbreak of the COVID-19 exhibits a positive impact on the global market. The growing investments by the pharmaceutical companies for the development of this validation technologies to fulfill the manufacturing standards executed by the regulatory bodies and accelerate the drug developments during and after the pandemic.
The COVID-19 swaps the focus of the organizations from the traditional cleaning procedures to advanced cleaning validation solutions like Process Analytical Technology (PAT) in the global market. Therefore, these factors reflect the pharmaceutical validation industry growth, which enables the expansion of the enterprises' product portfolio and supports them in the development of COVID-19 vaccines.
Know more about this report: request for sample pages
Industry Dynamics
Growth Drivers
The growing demand for the medical drug is directly associated with the growth of the pharmaceutical validation, which, in turn, impel the market growth globally. Factors such as poor health, increasing prevalence of disorders, growing incidences of acute & chronic diseases, and changing lifestyles need effective diagnostics and medicines for treatment.
In addition, manufacturers in biologics are investing in the development of the antibody drugs conjugates (ADCs) to medicine productions which are used for curing respiratory diseases, oncology, and inflammatory as well as cancer. The increasing approaches for the development of novel drugs also need the cleaning validation solution in the manufacturing services as governments are imposing regulatory standards.
Thereby, the development of medical drugs boosts the pharmaceutical cleaning validation industry demand for ensuring the safety of the products, checking impurities, and monitoring potential contamination.
Furthermore, growing policies in the pharmaceutical manufacturing industry for evading cross-contamination and spreading consciousness regarding the significance of the pharmaceutical validation process are further contributing to the global market development. Moreover, pharmaceutical companies have expanded their geographical footprints, impelling the pharmaceutical cleaning validation industry growth in the forecasting years.
Report Segmentation
The market is primarily segmented on the basis of product, test, and region.
|
By Product |
By Test |
By Region |
|
|
|
Know more about this report: request for sample pages
Insight by Type
The small molecule drug segment is the leading segment in terms of revenue generation in the year 2020 and is expected to dominate the pharmaceutical cleaning validation industry in approaching years owing to the highest production of small molecule drugs by the leading players. For instance, in 2018, the FDA approved nearly 59 novel drugs, including 71% small molecules and 29% biologics. Likewise, small molecules drugs are rising by 75% across the pharmaceutical cleaning validation industry.
Consequently, the growing production levels of small molecule drugs are positively influencing the growth of cleaning validation demand, which, in turn, leads to the dominance of the segment in the global market. The peptide segment is estimated to show substantial pharmaceutical cleaning validation growth in the global market. Peptide presents various advantages, such as it efficiently diffuses tissue owing to it is small size as well as less immunogenicity in comparison to recombinant antibodies and proteins at the biological level.
Also, peptides are manufactured at low costs with more activity per unit mass. As peptides are naturally stirring biologics and they are protected with better selectivity, efficiency, and specificity. Moreover, the rising number of approvals by authorized organizations and governments for peptide drugs for marketing endorsement is fueling the growth of the segment in the global market.
Insight by Test
The product-specific analytical tests segment is accounted for the largest revenue generator by holding the highest shares in the global pharmaceutical cleaning validation industry in 2020. The demand for High-performance Liquid Chromatography (HPLC) is increasing in the market because it offers various methods for detecting a photodiode array, UV, refractive index, fluorescence, evaporative light scattering, corona charged aerosol detection, as well as multiple types of swaps.
In addition, present segment features like peak separation, quantitative results, and mixed solvents for extraction solution can be selected—accordingly, these factors exhibit the growing demand of the segment in the global market. There is a rise in consumer base for the Total Organic Carbon (TOC) testing due to its features like time-saving and pocket-friendly tests, which positively influence market growth.
The operating price for the TOC test is lesser than HPLC tests by around 40-80%, along with a 70-80% faster analytical process than further conventional tests. Thus, these factors are anticipated to impelling the pharmaceutical cleaning validation industry growth in the approaching years.
Geographic Overview
Geographically, North America is the largest contributor of revenue in 2020 and is expected to dominate the global pharmaceutical cleaning validation market in the forecasting period. The increasing penetration of the cleaning validation by pharmaceutical manufacturing enterprises owing to the strict standards presented by the government and the FDA in the region leads to the dominance of the market.
Moreover, increasing spending on research and healthcare along with the presence of the major leading players shows noteworthy market growth across the region. Therefore, these factors create a productive demand for pharmaceutical cleaning validation in North America.
Moreover, the Asia Pacific is projected to grow at the highest CAGR across the globe in the approaching years. The growing expenditure on pharmaceutical cleaning, coupled with the increasing investment by international medical organizations, are some prominent factors for regional pharmaceutical cleaning validation market demand.
Additionally, the rising availability of healthcare facilities as well as the increasing existence of the pharmaceutical manufacturing plants attributes to the market growth over the forthcoming period. Accordingly, these factors are gaining huge prominence across the APAC region.
Competitive Landscape
Some of the Major Players operating in the global market include Avomeen, Hach, Intertek Group PLC, Kymos S.L., Lucideon Limited, Merck KGaA, ProPharma Group, QPharma, Inc., SGS SA, Shimadzu Corporation, Suez, Teledyne Tekmar, and Waters Corporation.
Pharmaceutical Cleaning Validation Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2020 |
USD 14.53 billion |
|
Revenue forecast in 2028 |
USD 22.29 billion |
|
CAGR |
5.6% from 2021 - 2028 |
|
Base year |
2020 |
|
Historical data |
2016 - 2016 |
|
Forecast period |
2021 - 2028 |
|
Quantitative units |
Revenue in USD billion and CAGR from 2021 to 2028 |
|
Segments covered |
By Product, By Test, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
|
Key Companies |
Avomeen, Hach, Intertek Group PLC, Kymos S.L., Lucideon Limited, Merck KGaA, ProPharma Group, QPharma, Inc., SGS SA, Shimadzu Corporation, Suez, Teledyne Tekmar, and Waters Corporation. |
