
Alport Syndrome Treatment Market Share, Size, Trends, Industry Analysis Report
By Product Type; By Disease Type; By Route Of Administration; By Distribution Channel (Online and Offline); By End User; By Region; Segment Forecast, 2023 - 2032
- Published Date:Jun-2023
- Pages: 117
- Format: PDF
- Report ID: PM3360
- Base Year: 2022
- Historical Data: 2019-2021
Report Outlook
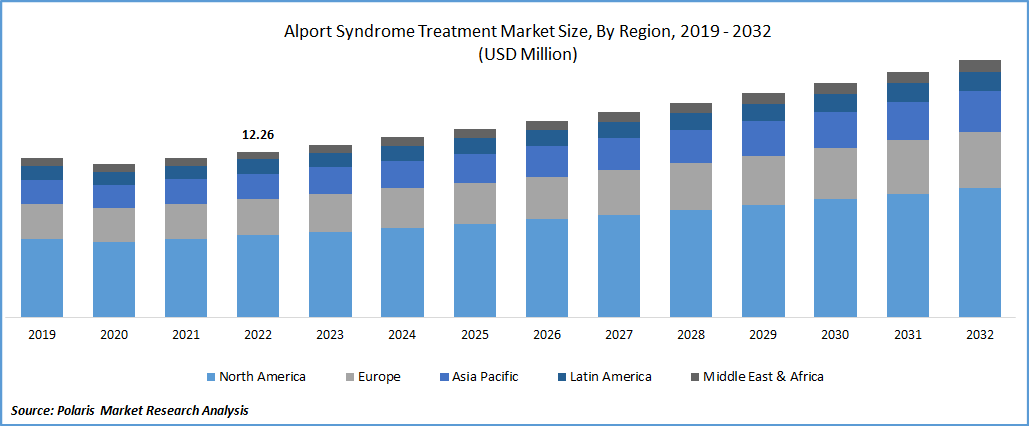
The global alport syndrome treatment market was valued at USD 12.26 million in 2022 and is expected to grow at a CAGR of 4.5% during the forecast period. The Alport syndrome causes anomalies in the inner ear, such as sensorineural hearing loss and vision loss. The rising prevalence of kidney disease is driving market growth. Kidney function declines as the illness develops, resulting in end-stage renal failure (ESRD). End-stage kidney disease (ESKD), which accounts for a significant amount of healthcare spending in the United States, is a prevalent condition that reduces patients' quality and duration of life. Diabetes and cardiovascular disease are frequently present in these individuals, and the mortality rate from cardiovascular disease is significant. Dialysis or kidney transplants are necessary for ESKD management.
To Understand More About this Research: Request a Free Sample Report
Further, most patients are first treated with hemodialysis, even though transplantation offers the largest improvement in survival and quality of life. As per the International Society of Nephrology, more than 850 million individuals worldwide suffer from some kidney illness, which is almost twice as many as the 422 million people who have diabetes and 20 times more than the 42 million people who have cancer globally or the 36.7 million people who have AIDS/HIV. Men are more likely than women to have CKD (10.4% vs. 11.8% globally). 13.3 million individuals worldwide encounter AKI each year, which may go away or develop into CKD or kidney failure in the future. Thus, the increasing prevalence of kidney diseases and dialysis is driving market growth.
Patients with Alport syndrome have problems in their inner ears, such as sensorineural hearing loss and vision loss. Men are also more prone than women to experience eye issues, hearing loss, and renal illness that worsens with time. While bilateral sensorineural hearing loss is unrelated to uremia, it has long been believed that renal failure inevitably follows, even though it may begin before the onset of end-stage chronic kidney disease. The course of renal failure and hearing loss would be parallel, and this fact would imply a dismal outlook for the kidneys.
Several clinics, ambulatory surgery centers, and hospitals are implementing cutting-edge technology. Some factors, including the rising incidence of chronic diseases, the quick advancements in the healthcare and medical fields, the high adoption of advanced tools and techniques, and the expanding funding from various public and private sectors, are largely responsible for the global market for Alport syndrome treatments' revenue growth.
Alport syndrome, a rare genetic disorder that affects roughly 60,000 people in the United States and 103,000 people in the European Union, is the second most frequent hereditary cause of kidney failure. Increases in white blood cell count, phosphorus, and ALT levels are risk factors for the severity of COVID-19 and mortality in patients on chronic hemodialysis. In hemodialysis patients, women are more likely to have COVID-19 than the general population.
Moreover, hemodialysis patients have a higher death rate. Those with prior comorbidities, such as those with various forms of renal illness, are disproportionately affected by COVID-19. All medical professionals, especially nephrology physicians, are challenged with quickly changing their practices to stop the virus' spread while still giving their patients the treatment they need to survive.
For Specific Research Requirements, Speak With the Research Analyst
Industry Dynamics
Growth Drivers
Growing genetic condition, prevalence is a significant element influencing alport syndrome treatment market revenue growth. There is no known treatment for Alport syndrome, a primary cause of renal damage in individuals. The availability of medications to treat its symptoms, such as high blood pressure, is therefore anticipated to fuel the expansion of the global market. For instance, in January 2022, A world-class research program will be implemented owing to a partnership with Kidney Research UK and a £2.55 million contribution from the Stoneygate Trust. Throughout the forecast period, growing expenditures in research & development activities, rising need for precision medicine, increased adoption of home care settings and point-of-care diagnostics, and rising focus on medication development are anticipated to propel worldwide market revenue growth.
Moreover, a person with Alport syndrome must be identified, diagnosed, and managed by their primary care physician (PCP). The primary care provider (PCP), also known as a general pediatrician, family physician, or nurse practitioner, is typically the first health care provider to identify and perform an initial assessment of key Alport syndrome symptoms, such as hematuria and hearing loss, and then refers patients to nephrologists, audiologists for the additional evaluation and management.
Report Segmentation
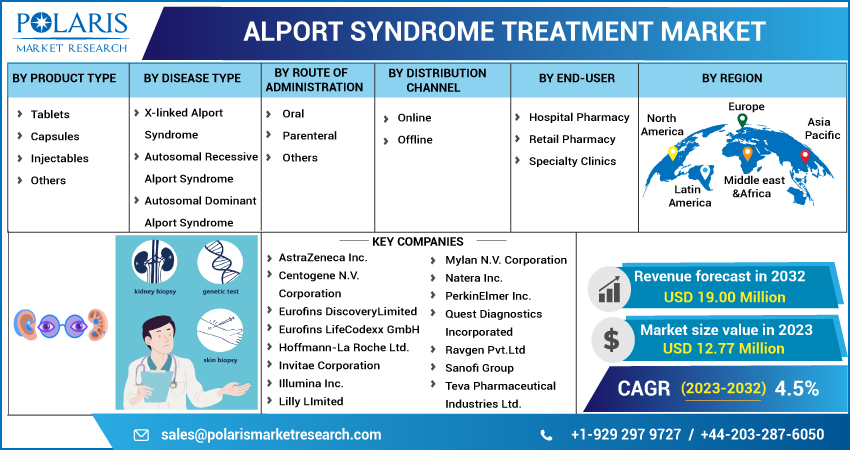
The market is primarily segmented based on product type, disease type, route of administration, distribution channel, end-user, and region.
|
By Product Type |
By Disease Type |
By Route Of Administration |
By Distribution Channel |
By End User |
By Region |
|
|
|
|
|
|
To Understand the Scope of this Report: Speak to Analyst
The tablets segment is expected to grow fastest over the projected period.
The tablets segment is poised to grow significantly in the market. This can be attributed to several factors. Firstly, tablets offer patients convenience and ease of administration, improving medication adherence. Secondly, advancements in formulation technology have led to the development of innovative and effective tablet formulations for Alport Syndrome treatment.
Thirdly, healthcare providers’ and patients' increasing preference for oral drug delivery systems further drives the demand for tablets. Moreover, tablets often offer cost-effective options compared to other treatment modalities, making them more accessible to a larger patient population. These factors contribute to the tablets segment being projected as the fastest-growing segment in the alport syndrome treatment market.
The X-linked Alport Syndrome is expected to hold a significant revenue share over the forecast period
A genetic investigation of the COL4 genes confirms the presence of X-linked Alport syndrome, hypothesized based on clinical features and a family history of hematuria, deafness, and renal impairment. If genetic testing is unclear or not accessible, a histological assessment of the kidney is helpful.
Furthermore, kidney and skin samples can be used for immunohistochemical tests, and basement membrane ultrastructure can be analyzed using electron microscopy. Type IV collagen chains' aberrant expression patterns support the diagnosis, but the normal expression does not rule it out. A biopsy of the afflicted tissue is required for the diagnosis of leiomyomatosis. Lesions and the concomitant distortion of the afflicted tract can be seen using radiological imaging.
The demand in North America is expected to grow significantly over the projected period.
The North American market is anticipated to have the greatest market share. This is because of the condition's rising prevalence, modern healthcare systems, and the presence of testing firms. In addition, a vast network of laboratories and sophisticated diagnostic procedures are anticipated to propel the alport syndrome market in North America. The alport syndrome market in this area is also expected to grow due to the key players' strong R&D efforts and extensive product pipelines. Thirty-seven million adult Americans are thought to have chronic kidney disease (CKD), and 90% are unaware of their condition. Chronic kidney disease affects one in three persons in the United States.
Additionally, Asia Pacific dominated the regional market in 2022 and accounted for the largest revenue share with Increasing healthcare costs, numerous government initiatives and CKD awareness campaigns, the availability of cutting-edge products, and the presence of newly built, state-of-the-art healthcare facilities in the region, The two main risk factors for developing kidney disease are diabetes and high blood pressure. According to estimates, Africa and the Asia-Pacific area contain the world's biggest populations of people with diabetes and high blood pressure.
Competitive Insight
Some of the major players operating in the global market include AstraZeneca, Centogene, Eurofins Discovery, Eurofins LifeCodexx, Roche Ltd., Invitae Corporation, Illumina, Lilly Limited, Mylan, Natera, PerkinElmer, Quest Diagnostics, Ravgen, Sanofi Group, and Teva Pharmaceutical.
Recent Developments
- In September 2022, a Phase II clinical trial investigation was carried out on hydroxychloroquine's safety, tolerability, and effectiveness in eligible kids with Alport syndrome at Shanghai Children's Hospital.
- In August 2022, The National Kidney Foundation (NKF) and the Alport Syndrome Foundation (ASF) formed a new cooperation to help the thousands of people with the devastating Alport syndrome, a rare hereditary kidney illness, and their families.
Alport Syndrome Treatment Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2023 |
USD 12.77 million |
|
Revenue forecast in 2032 |
USD 19.00 million |
|
CAGR |
4.5% from 2023- 2032 |
|
Base year |
2022 |
|
Historical data |
2019- 2021 |
|
Forecast period |
2023- 2032 |
|
Quantitative units |
Revenue in USD million and CAGR from 2023 to 2032 |
|
Segments Covered |
By Product Type, By Disease Type, By Route of administration, By Distribution Channel, By End User, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America; Middle East & Africa |
|
Key Companies |
AstraZeneca Inc., Centogene N.V. Corporation, Eurofins Discovery Limited, Eurofins LifeCodexx GmbH, Hoffmann-La Roche Ltd., Invitae Corporation, Illumina Inc., Lilly LImited, Mylan N.V. Corporation, Natera Inc., PerkinElmer Inc., Quest Diagnostics Incorporated, Ravgen Pvt.Ltd, Sanofi Group, and Teva Pharmaceutical Industries Ltd. |
FAQ's
key companies in alport syndrome treatment market are AstraZeneca, Centogene, Eurofins Discovery, Eurofins LifeCodexx, Roche Ltd., Invitae Corporation, Illumina, Lilly Limited, Mylan.
The global alport syndrome treatment market expected to grow at a CAGR of 4.5% during the forecast period.
The alport syndrome treatment market report covering key segments are product type, disease type, route of administration, distribution channel, end-user, and region.
key driving factors in alport syndrome treatment market are increase prevalence of alport syndrome.
The global alport syndrome treatment market size is expected to reach USD 19.00 million by 2032.
