
Clinical Trial Kits Market Share, Size, Trends, Industry Analysis Report
By Service (Kitting Solutions, By Phase (Phase I, Phase II, Phase III and Phase IV), By Region; Segment Forecast, 2025 - 2034
- Published Date:Sep-2025
- Pages: 110
- Format: PDF
- Report ID: PM2177
- Base Year: 2024
- Historical Data: 2020 - 2023
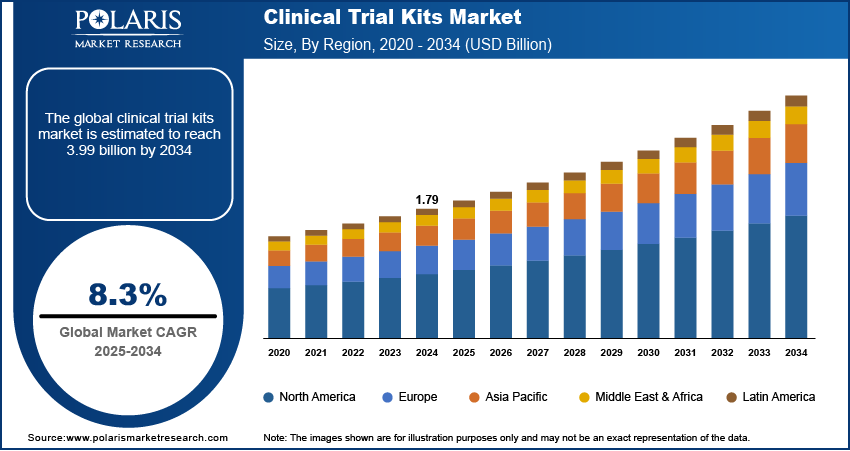
The global clinical trial kits market was valued at USD 1.79 billion in 2024 and is estimated to grow at a CAGR of 8.3% during the forecast period. The market is anticipated to grow on account of the growing number of clinical trials across the globe. Clinical testing kits enable the patient to collect & submit their samples directly to the labs for testing, in turn eliminating the need for healthcare professionals to oversee sample collection, packaging & shipping.
Key Insights
- Logistics is the leading segment during the forecast period. This is driven by the growing demand for efficient, temperature-controlled transportation of sensitive clinical trial materials.
- Phase III dominated the segment in 2024. This is due to rising patient volumes drive demand for comprehensive trial kits and services.
- North America dominated the global market in 2024 due to robust pharmaceutical R&D activity.
- Asia Pacific is projected to exhibit a significant CAGR over the anticipated period. This is driven by expanding healthcare infrastructure across emerging economies.
Industry Dynamics
- The market is driven by the rising number of clinical trials across various therapeutic areas globally.
- Innovations in decentralized trials and direct-to-patient kit distribution models drive market growth over the forecast period.
- Increasing investments by pharmaceutical and biotech companies in drug development fuel market revenue.
- Industry is expanding due to growing focus on patient-centric trials and real-time data collection.
Market Statistics
- 2024 Market Size: USD 1.79 Billion
- 2034 Projected Market Size: USD 3.99 Billion
- CAGR (2025-2034): 8.3%
- North America: Largest Market Share
To Understand More About this Research: Request a Free Sample Report
AI Impact on Clinical Trial Kits Market
- AI helps to enhance patient data analysis accuracy, enabling faster and more reliable identification of suitable clinical trial candidates.
- AI helps to optimize supply chain logistics and improving overall efficiency of clinical trial kit distribution.
- AI helps in real-time monitoring of patient data.
- AI helps to detect anomalies in clinical data patterns.
The companies are moving forward to provide direct-to-consumer testing services. Various aspects of the testing process, including test ordering to sample collection, are making their way into the patient’s houses. Additionally, government bodies across the globe are increasing their investments in R&D activities, and clinical testing focused on various health conditions. This is anticipated to boost the growth of the clinical trial kits market across the globe.
The widespread COVID-19 witnessed a positive impact on the market, owing to the rising popularity of home diagnostic solutions across the globe. These solutions help in reducing the high workloads that healthcare professionals are facing with patient monitoring and day-to-day testing. Moreover, the Covid-19 outbreak has resulted in a progressive use of sample collection kits on account of the continuity of these testing and enhanced focus on patient safety. This has further accelerated the implementation of patient-centric sampling strategies in clinical testing.
Though the Covid-19 outbreak has slowed the development and damaged the clinical trial quality, the elimination of laboratory assessments and non-essential visits along with the sample collection & diagnostic testing has allowed the market of these kits to grow while ensuring patient safety during the pandemic. In March 2021, the Global Data’s Pharma Intelligence Center stated that over 1,200 medical trials globally experienced disruption due to the Covid-19 outbreak.

Industry Dynamics
Growth Drivers
The major driving factors for clinical trial kits market growth are the increasing number of clinical trials, growing demand for remote services, along the rising clinical trial complexity. The increasing R&D investments in medical trial kits supplies, along with the rising investments in the healthcare industry, are further estimated to drive the market growth of these kits. The growing R&D expenditure of biopharmaceutical & pharmaceutical companies is estimated to lead to an increase in the number of trials that are held globally.
According to the PhRMA (Pharmaceutical Research & manufacturers of America), all the PhRMA members are increasing their investment and drug development efforts. The coronavirus outbreak boosted the growth on account of the rising popularity of remote testing and observation for day-to-day testing and patient monitoring. At present, remote clinical trial services are an essential part of patient retention, safety & satisfaction. The increasing demand for home testing is further enabling essential medical research to continue remotely. The increasing quality, along with the decreasing cost of remote testing, is anticipated to drive the growth of these kits over the coming years.
Report Segmentation
The market is primarily segmented on the basis of service, phase, and region.
|
By Service |
By Phase |
By Region |
|
|
|
Know more about this report: request for sample pages
Insight by Service
Logistics is the leading segment accounting for the highest share in the global market of these kits. At present, there is a huge growth in the demand for simplification of the overall logistics process in medical trials across the globe. Pharmaceutical companies are joining hands with various leading logistics partners across the globe in order to provide remote services.
The logistic companies carefully select & monitor their performance, assuring on-time quality services along with around-the-clock monitoring. Various authorities globally are becoming stringent in terms of the quality of GCP (Good clinical research practice), packaging, and storage. This stringent regulatory environment is boosting the growth of the segment.
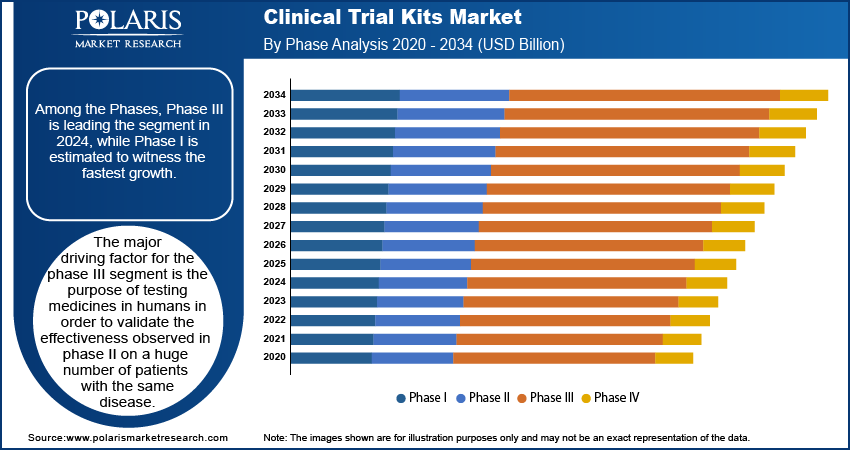
Insight by Phase
Among the Phases, Phase III is leading the segment in 2024, while Phase I is estimated to witness the fastest growth. The major driving factor for the phase III segment is the purpose of testing medicines in humans in order to validate the effectiveness observed in phase II on a huge number of patients with the same disease. Phase III demonstrates the safety and effectiveness of a new medicine or vaccine.
Various pharmaceutical products that have successfully concluded phase II clinical trials fail in phase III, generally on account of the side effects of therapeutic effectiveness. The segment is further driven by the increasing per capita income in the developed & developing countries. The rising number of patients with rare diseases is further propelling the demand for phase III clinical trials. The phase III clinical trials require a higher need for outsourcing services when compared to Phase I & phase II, owing to the larger patient pool.
Geographic Overview
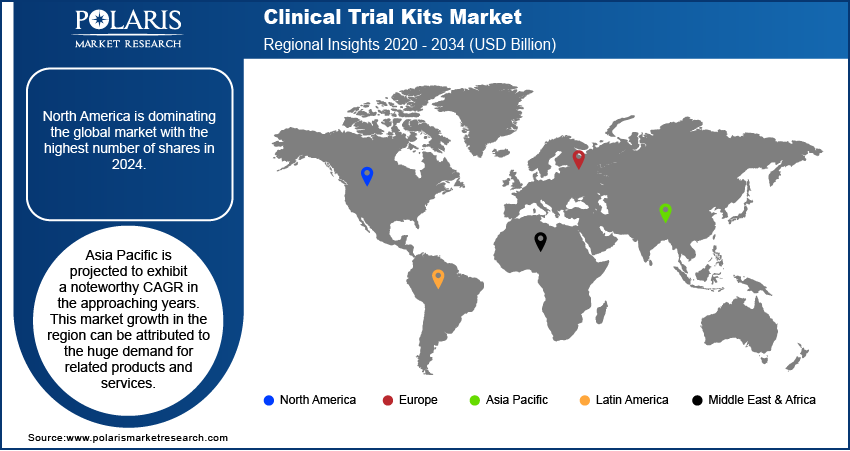
Geographically, North America is dominating the global market with the highest number of shares in 2024. North America is the primary adopter of developed or modern technologies, as well as it is the chief hub of several major players in the market. Most large pharmaceutical companies are located in the United States and perform the majority of their business across this region. Along with these factors, the favorable government efforts in the region are further estimated to drive the growth of the industry in the region.
Asia Pacific is projected to exhibit a noteworthy CAGR in the approaching years. This market growth in the region can be attributed to the huge demand for related products and services. Asia Pacific region is one the fastest growing pharmaceutical marketplaces globally, presenting huge growth opportunities for the clinical trial kits market over the coming years. Furthermore, Asia Pacific is the most populated region, thus having an enormous patient pool, the majority of which do not have access to inexpensive medical alternatives. Therefore, these factors foster the industry growth in the Asia Pacific region over the upcoming scenario.
Competitive Insight
Some of the major players operating the global market include Almac Group, Alpha Laboratories, Brooks Life Science, Cerba research, Charles River Laboratories, Clinigen, LabConnect, Labcorp Drug Development, Marken a UPS company, Patheon, Precision Medicine Group, Q2 Solutions.
Clinical Trial Kits Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2024 |
USD 1.79 billion |
| Market size value in 2025 | USD 1.94 billion |
|
Revenue forecast in 2034 |
USD 3.99 billion |
|
CAGR |
8.3% from 2025 - 2034 |
|
Base year |
2024 |
|
Historical data |
2020 - 2023 |
|
Forecast period |
2025 - 2034 |
|
Quantitative units |
Revenue in USD million and CAGR from 2025 to 2034 |
|
Segments covered |
By Service, By Phase, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America; Middle East & Africa |
|
Key Companies |
Almac Group, Alpha Laboratories, Brooks Life Science, Cerba research, Charles River Laboratories, Clinigen, LabConnect, Labcorp Drug Development, Marken a UPS company, Patheon, Precision Medicine Group, Q2 Solutions |
FAQ's
• The global market size was valued at USD 1.79 billion in 2024 and is projected to grow to USD 3.99 billion by 2034.
• The global market is projected to register a CAGR of 8.3x% during the forecast period.
• North America dominated the market in 2024.
• A few of the key players in the market are Almac Group, Alpha Laboratories, Brooks Life Science, Cerba research, Charles River Laboratories, Clinigen, LabConnect, Labcorp Drug Development, Marken a UPS company, Patheon, Precision Medicine Group, Q2 Solutions.
• The logistics segment is expected to hold largest market share during the forecast period.
