
Europe Rare Disease Diagnostics Market Share, Size, Trends, Industry Analysis Report
By Disease Type, By Product Type, By Sample Type, By Technology, By Test, By Age Group, By Trait Type, By End-User, By Country; Segment Forecast, 2024 - 2032
- Published Date:May-2024
- Pages: 114
- Format: PDF
- Report ID: PM4900
- Base Year: 2023
- Historical Data: 2019-2022
Report Outlook
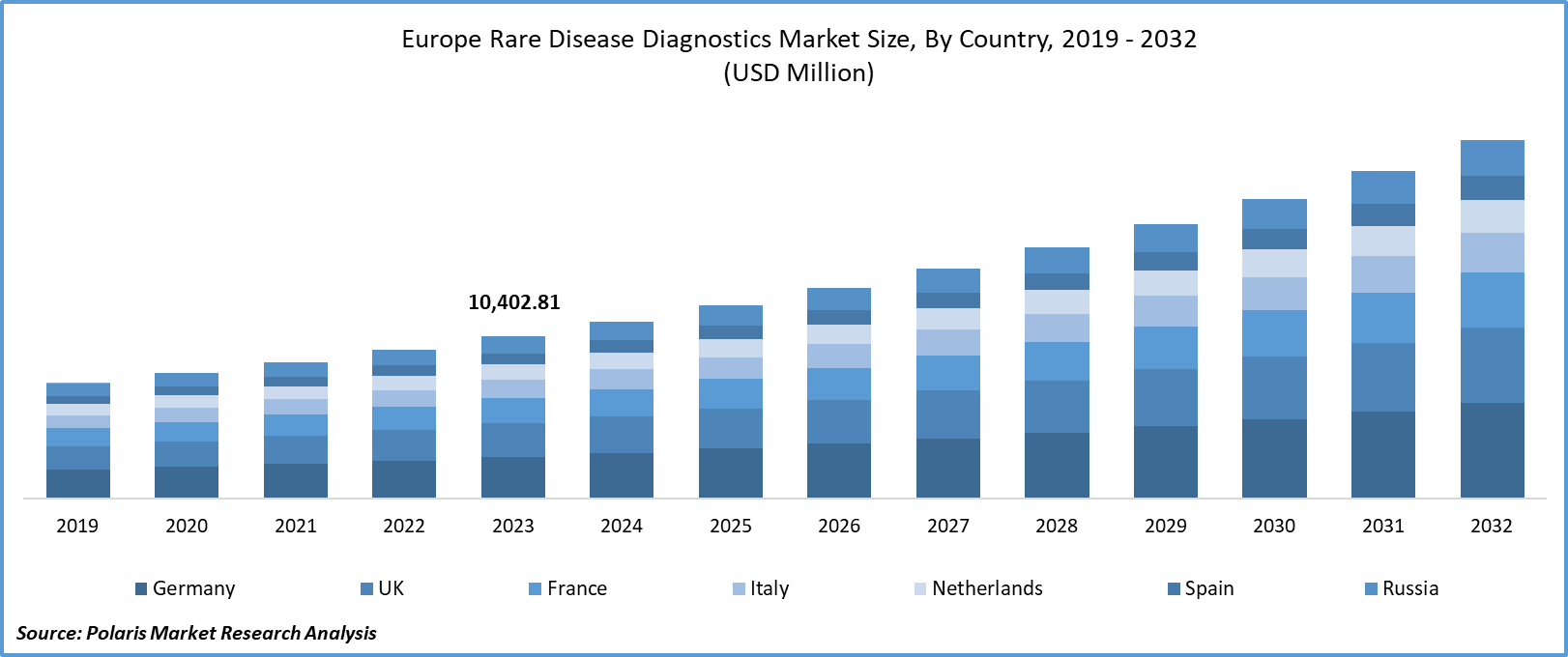
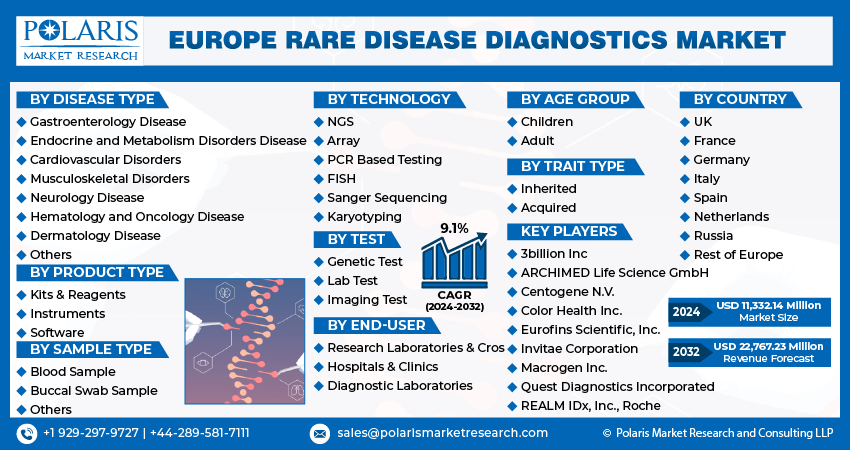
Europe Rare Disease Diagnostics market size was valued at USD 10,402.81 million in 2023. The market is anticipated to grow from USD 11,332.14 million in 2024 to USD 22,767.23 million by 2032, exhibiting the CAGR of 9.1% during the forecast period.
Industry Trends
The Europe Rare Disease Diagnostics market has witnessed significant growth in recent years, driven by enhanced availability of diagnosis, information, and care for patients. This objective is achieved by consolidating limited resources distributed throughout the EU, which facilitates the exchange of expertise and information among patients and healthcare professionals. The European approach can be best described as a combination of essential components such as encouraging the establishment of national plans and strategies specifically designed for rare diseases, establishing and providing support to European reference networks, which serve as networks of healthcare professionals specializing in rare diseases, supporting the identification, classification, and compilation of rare diseases, assisting in the authorization and designation of orphan medicinal products, expanding and enriching the knowledge base through research initiatives, empowering patient organizations to actively participate in decision-making processes.
To Understand More About this Research:Request a Free Sample Report
ERNs are networks that connect healthcare providers from across Europe in order to facilitate discussions on complex and rare diseases and conditions that necessitate specialized treatment. These networks work together to assess patients' diagnoses and treatment plans by utilizing virtual advisory panels comprised of medical specialists from various disciplines. This collaboration is made possible through a dedicated IT platform. Additionally, ERNs actively engage in research, establish registries, develop clinical guidelines, and promote the exchange of knowledge and expertise among healthcare professionals and patient organizations.
Key Takeaways
- Germany dominated the market and contributed over 9.7% of the CAGR.
- By disease type, the Hematology and Oncology Disease segment accounted for the largest Europe Rare Disease Diagnostics market share.
- By product type, the kits and reagents segment is anticipated to grow with a lucrative CAGR over the Europe Rare Disease Diagnostics market forecast period.
What are the market drivers driving the demand for market?
Rise in adoption of technologies drive the European Rare Disease Diagnostics market growth.
The market is experiencing significant growth as there is progress in information technology, specifically in artificial intelligence (AI) and machine learning, which plays a crucial role in enhancing the outcomes for individuals with rare diseases. The integration of AI and machine learning in healthcare and medicine is becoming increasingly prevalent. For instance, the development of online tools for diagnosing genetic or rare diseases, which utilize the concept of phenotype to construct diagnostic models through phenotypic similarity and machine learning algorithms number of data sources like hospital information systems, electronic health records, and health-related registries, a new strategy is essential for creating AI-driven decision support tools to aid clinicians in their diagnostic processes and reduce the time taken for rare disease patients to receive a diagnosis. The objective is to pinpoint the main obstacles in aligning European rare disease databases, particularly concerning ML-based screening technologies, with regard to organizational and legal standards.
Which factor is restraining the demand for market?
Limited awareness affects the European market growth.
Limited awareness of the rare disease diagnostics market in Europe presents a significant obstacle to its growth and effectiveness. Despite the growing recognition of rare diseases, there is still a general lack of awareness among healthcare professionals and the public regarding the breadth and complexity of these conditions and the availability of specialized diagnostic tools and services. This lack of awareness leads to delays in diagnosis, misdiagnosis, and underdiagnosis of rare diseases, which ultimately affect patient outcomes and increase healthcare costs.
Report Segmentation
The market is primarily segmented based on disease type, product type, sample type, technology, test, age group, trait type, end-user and country.
|
By Disease Type |
By Product Type |
By Sample Type |
By Technology |
By Test |
By Age Group |
By Trait Type |
By End-User |
By Country |
|
|
|
|
|
|
|
|
|
To Understand the Scope of this Report:Speak to Analyst
Category Wise Insights
By Disease Type Insights
Based on disease type analysis, the market is segmented gastroenterology disease, endocrine and metabolism disorders disease, cardiovascular disorders, musculoskeletal disorders, neurology disease, hematology and oncology disease, dermatology disease, others. The dominance of the Hematology and Oncology Disease segment in Europe's Rare Disease Diagnostics market due to the rise in number of treatment options, advancements in tools, access across the European Union to precise diagnostics that are of high quality, reproducible, and affordable in order to achieve genuine personalized medicine. This will lead to enhanced, customized treatment options that reduce side effects, improve patients' quality of life, and increase cost efficiency for society. Patient immunophenotyping is conducted in conjunction with molecular profiling and identification of disease-specific biomarkers and genetic signatures. Additionally, patient prognosis, treatment stratification, and trial eligibility are assessed and monitored to uphold healthcare standards. Upon the detection of a multidisciplinary tumor, the diagnosis is verified and the treatment plan is subsequently reviewed with the patient.
By Product Type Insights
Based on product type analysis, the market has been segmented into kits & reagents, instruments, and software. The kits and reagents segment dominated the market and is expected to experience significant growth with a lucrative CAGR over the forecast period in Europe's Rare Disease Diagnostics market owing to providing a streamlined process and reliable data quality testing to guarantee cost-effective and equitable accessibility for all. However, integration of bioinformatics in kits and reagents will allow higher diagnostic yields and a faster time to diagnosis at lower overall costs. For instance, CENTOGENE offers automated bioinformatics processes NGS data using approved testing products enriched with information from the rare disease-focused Bio-Databank. With AI-assisted clinical analysis and medical reporting, CentoCloud ensures consistently high standards and precise diagnostic results.
Country-wise Insights
Germany
Germany's leading position in the rare disease diagnostics market can be attributed to several factors. To begin with, the country possesses a strong healthcare infrastructure, distinguished research institutions, and a commitment to innovation, all of which contribute to the advancement of diagnostic technologies. Moreover, Germany's regulatory environment is supportive, and ample funding opportunities encourage both public and private investments in rare disease diagnostics. Additionally, Germany's strategic location within Europe enables fruitful collaborations with neighboring countries, facilitating the exchange of knowledge and resources.
Competitive Landscape
The competitive landscape for the European Rare Disease Diagnostics market is characterized by intense rivalry among key players striving to gain market share and differentiate themselves through innovation and strategic partnerships. Amidst this landscape, collaboration between technology vendors, cloud service providers, and industry stakeholders remains crucial for driving innovation, expanding market reach, and addressing evolving customer demands in the European Rare Disease Diagnostics market.
Some of the major players operating in the European market include:
- 3billion Inc
- ARCHIMED Life Science GmbH
- Centogene N.V.
- Color Health Inc.
- Eurofins Scientific, Inc.
- Invitae Corporation
- Macrogen Inc.
- Quest Diagnostics Incorporated
- REALM IDx, Inc.
- Roche
Recent Developments
- ·In March 2024, Oxford Nanopore Technologies entered into a collaboration with SeqOne, an artificial intelligence developer, to bolster its clinical diagnostic testing through next-generation sequencing. The objective is to establish a comprehensive platform that utilizes extended nanopore sequencing reads for complete genome analysis. Initially, the focus will be on detecting genetic variations linked to rare diseases, with plans to later expand the initiative to encompass cancer testing.
- ·In April 2024, Ipsen, a biopharmaceutical firm headquartered in Paris, and Skyhawk, a clinical-stage company, entered into an exclusive partnership to explore and create small molecule treatments that target RNA for the purpose of addressing rare neurological disorders.
Report Coverage
The Europe rare disease diagnostics market report emphasizes on key countries across the region to provide better understanding of the product to the users. Also, the report provides market insights into recent developments, trends and analyzes the technologies that are gaining traction around the region. Furthermore, the report covers in-depth qualitative analysis pertaining to various paradigm shifts associated with the transformation of these solutions.
The report provides detailed analysis of the market while focusing on various key aspects such as competitive analysis, disease type, product type, sample type, technology, test, age group, trait type, end-user and their futuristic growth opportunities.
Europe Rare Disease Diagnostics Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2024 |
USD 11,332.14 million |
|
Revenue forecast in 2032 |
USD 22,767.23 million |
|
CAGR |
9.1% from 2024 – 2032 |
|
Base year |
2023 |
|
Historical data |
2019 – 2022 |
|
Forecast period |
2024 – 2032 |
|
Quantitative units |
Revenue in USD million and CAGR from 2024 to 2032 |
|
Segments covered |
By Disease Type, By Product Type, By Sample Type, By Technology, By Test, By Age Group, By Trait Type, By End-User, By Country |
|
Customization |
Report customization as per your requirements with respect to countries, region and segmentation. |
FAQ's
key companies in Europe Rare Disease Diagnostics Market are 3billion Inc., ARCHIMED Life Science GmbH, Centogene, Color Health Inc., Eurofins Scientific, Inc.
Europe Rare Disease Diagnostics market exhibiting the CAGR of 9.1% during the forecast period.
The Europe Rare Disease Diagnostics Market report covering key segments are disease type, product type, sample type, technology, test, age group, trait type, end-user and country
key driving factors in Europe Rare Disease Diagnostics Market are Rise in adoption of technologies
The Europe Rare Disease Diagnostics market size is expected to reach USD 22,767.23 Million by 2032
