
Regulatory Information Management System Market Share, Size, Trends, Industry Analysis Report
By Component (Software, Services); By Deployment; By Enterprise Size; By Application; By End Use; By Region; Segment Forecast, 2024 - 2032
- Published Date:Jun-2024
- Pages: 115
- Format: PDF
- Report ID: PM4929
- Base Year: 2023
- Historical Data: 2019-2022
Report Outlook
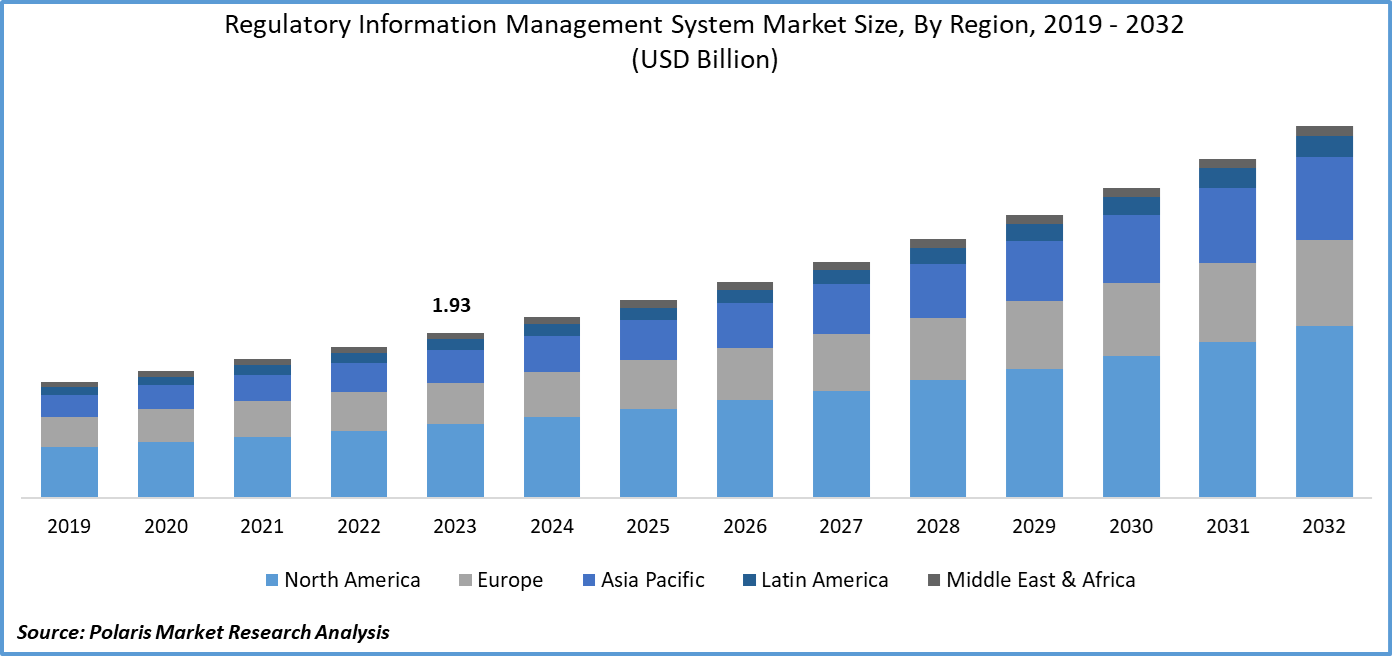
Regulatory Information Management System Market size was valued at USD 1.93 billion in 2023. The market is anticipated to grow from USD 2.11 billion in 2024 to USD 4.35 billion by 2032, exhibiting the CAGR of 9.5% during the forecast period.
Industry Trends
Regulatory Information Management System (RIMS) is a software solution designed to streamline and organize regulatory information related to industries such as pharmaceuticals, healthcare, and biotechnology. It serves as a centralized repository for storing, managing, and tracking regulatory documents, submissions, and compliance data. RIMS helps companies adhere to complex regulatory requirements by providing functionalities for document authoring, version control, submission planning, and regulatory tracking.
To Understand More About this Research:Request a Free Sample Report
The Regulatory Information Management System (RIMS) market is experiencing robust growth driven by the increasing complexity of regulatory requirements across various industries such as pharmaceuticals, biotechnology, medical devices, and healthcare. As regulatory bodies worldwide continuously evolve their guidelines and compliance standards, companies are increasingly turning to RIMS solutions to streamline their regulatory processes and ensure compliance. Also, the growing adoption of electronic submissions and the digitization of regulatory documentation further fuel the demand for RIMS solutions.
The primary market trend in the market is the integration of advanced technologies such as artificial intelligence (AI) and machine learning (ML) to enhance regulatory processes. These technologies enable automation of routine tasks, predictive analytics for better decision-making, and advanced data analysis for compliance insights. Cloud-based RIMS solutions are gaining traction due to their scalability, flexibility, and cost-effectiveness, allowing organizations to manage regulatory information securely from anywhere and at any time.
The driving factors propelling the growth of the RIMS market include the increasing focus on patient safety and drug efficacy, stringent regulatory requirements, and the need for efficient management of regulatory documentation and submissions. The rise in the number of clinical trials, drug approvals, and product launches necessitates effective regulatory management solutions to navigate the complex regulatory landscape and expedite time to market. However, the market is hindered by data security concerns, especially with the handling of sensitive regulatory information and the high initial investment required for implementing RIMS solutions.
Key Takeaways
- North America dominated the market and contributed over 41% market share of the regulatory information management system market size in 2023
- By component category, the software segment dominated the global regulatory information management system market size in 2023
- By end-use category, the pharmaceutical industry segment is projected to grow with a significant CAGR over the regulatory information management system market forecast period
What are the market drivers driving the demand for the market?
Increasing Complexity of Regulatory Requirements is driving regulatory information management system market growth.
Regulatory bodies worldwide are continually updating and expanding their guidelines and compliance standards, necessitating organizations to navigate a dynamic and intricate regulatory landscape. Also, industries such as pharmaceuticals, healthcare, and biotechnology are facing heightened scrutiny and stringent regulations to ensure patient safety, product efficacy, and data integrity. As a result, companies seek comprehensive RIMS solutions to streamline regulatory processes, manage documentation, and ensure compliance with evolving regulations.
RIMS provides a centralized platform for organizations to efficiently track, manage, and report regulatory information, enabling them to adapt to changing requirements and mitigate compliance risks effectively. Thus, the increasing complexity of regulatory requirements acts as a catalyst for the adoption of RIMS solutions, driving market growth as organizations prioritize regulatory compliance and efficiency in their operations.
Which factor is restraining the demand for a Regulatory Information Management System?
Data security concerns and high initial investments are hindering the regulatory information management system market growth.
Handling sensitive regulatory information raises apprehensions regarding data security and privacy breaches, particularly considering the strict regulatory requirements governing data protection. Many organizations are hesitant to rely on external RIMS due to security concerns. They fear that the lack of robust security measures in place could lead to data breaches and compromise sensitive regulatory information.
Also, the high initial investments required for implementing RIMS solutions pose a financial barrier, especially for small and medium enterprises. The upfront costs associated with software licensing, infrastructure setup, and employee training can deter organizations from committing to RIMS solutions despite recognizing their potential benefits in regulatory compliance and efficiency. Therefore, data security concerns and high initial investments impede market growth.
Report Segmentation
The market is primarily segmented based on component, deployment, enterprise size, application, end-use, and region.
|
By Component |
By Deployment |
By Enterprise Size |
By Application |
By End Use |
By Region |
|
|
|
|
|
|
To Understand the Scope of this Report:Speak to Analyst
Category Wise Insights
By Component Insights
Based on component category analysis, the market has been segmented on the basis of software and services. The software segment dominated the global regulatory information management system market size in 2023 since software solutions offer a comprehensive suite of functionalities tailored specifically for managing regulatory information, including document authoring, version control, submission planning, and compliance tracking. These software platforms provide organizations with the flexibility to customize their regulatory workflows and adapt to evolving regulatory requirements efficiently.
Also, the increasing digitization of regulatory processes and the transition towards electronic submissions have propelled the demand for software-based RIMS solutions, driving market growth. Advancements in technology, such as cloud-based deployment options, have further enhanced the capabilities and appeal of software-based RIMS solutions, solidifying their dominance in the market segment.
By End Use Insights
Based on end-use category analysis, the market has been segmented on the basis of the pharmaceutical industry, biotechnology industry, and clinical research organizations. The pharmaceutical industry segment is anticipated to experience significant growth over the forecast period in the market since the pharmaceutical sector operates within a highly regulated environment, with stringent requirements imposed by regulatory bodies worldwide to ensure drug safety, efficacy, and quality. As regulatory standards continue to evolve and become more complex, pharmaceutical companies face mounting pressure to streamline their regulatory processes and ensure compliance with changing requirements. Hence, there is a growing recognition of the need for comprehensive RIMS solutions within the pharmaceutical industry to manage regulatory documentation, submissions, and compliance activities effectively. In addition, the increasing number of drug approvals, clinical trials, and product launches in the pharmaceutical sector further drives the demand for RIMS solutions.
Regional Insights
North America
In 2023, North America dominated the global market since the region boasts a robust regulatory environment, particularly in industries such as pharmaceuticals, healthcare, and biotechnology, which necessitates comprehensive regulatory compliance solutions. As a result, organizations in the region prioritize investments in RIMS solutions to navigate the complex regulatory landscape effectively and ensure adherence to stringent compliance standards. Also, North America is home to a large number of leading RIMS solution providers and technology innovators, fostering a competitive market ecosystem that drives innovation and advancements in regulatory management software. The presence of key pharmaceutical and life sciences companies in North America, along with the region's significant healthcare expenditure and focus on research and development, contributes to the high demand for RIMS solutions to support regulatory processes and enhance operational efficiency.
Asia Pacific
The Asia Pacific region is poised for substantial growth in the global market since the region is witnessing rapid economic growth, coupled with increasing investments in several industries. As these industries expand and mature, there is a growing emphasis on regulatory compliance to ensure product safety, efficacy, and quality, driving the demand for RIMS solutions. Along with this, regulatory bodies in countries across the Asia Pacific region are increasingly aligning their standards and requirements with international regulations, necessitating organizations to adopt comprehensive RIMS solutions to navigate the evolving regulatory landscape effectively. The rising adoption of digital technologies and the proliferation of electronic submissions in regulatory processes further fuel the demand for RIMS solutions in the region.
Competitive Landscape
The market's competitive landscape is facing intense competition, in which key players are striving to enhance their market presence. Leading vendors in the RIMS market are engaged in strategies such as mergers and acquisitions, partnerships, collaborations, and product innovations to expand their product portfolios, broaden their geographical reach, and meet customers' evolving needs. Also, regional players are increasingly focusing on catering to the specific regulatory requirements of local markets, further intensifying competition in the global RIMS market.
Some of the major players operating in the global market include:
- AmpleLogic
- ArisGlobal
- Calyx
- DDi
- Ennov
- Ithos Global Inc.
- Kalypso (Rockwell Automation)
- Körber AG
- LORENZ Life Sciences Group
- MasterControl Solutions, Inc
- Phlexglobal
- Rimsys
- Veeva Systems
Recent Developments
- In May 2024, Rimsys, a medtech regulatory information management software provider, announced the beta launch of Rimsys Intel. It is a centralized hub for global regulatory intelligence data that is community-driven. Rimsys Intel offers free access to regulatory intelligence, including regulatory affiliations, legislation, UDI requirements, risk class information for medical devices and IVDs, as well as market access requirements for each regulated country.
- In May 2022, Rimsys launched Rimsys 5 at the MedTech Forum held in Barcelona, Spain. This latest version of RIM software is specifically designed to assist MedTech companies in enhancing their compliance capabilities and expediting their product launch process.
- In November 2019, IQVIA has launched IQVIA RIM Smart, which is a fully integrated, cloud-based regulatory information management solution for the life sciences industry. This new solution is designed to use advanced artificial intelligence (AI) and machine learning (ML) technologies to enable intelligent management of the entire regulatory lifecycle of a product portfolio.
- In May 2021, Calyx, the provider of eClinical and Regulatory solutions and services, launched Calyx RIM v7.0, the latest version of its Regulatory Information Management (RIM) system.
- In February 2023, ArisGlobal, a provider of life sciences software, introduced its latest offering, Investigational Product RIMS. This platform is specifically designed to cater to the distinctive requirements of life sciences and medical device firms as they navigate through the investigational stages of drug development. It is integrated with the end-to-end technology platform LifeSphere, which automates various core drug development functions.
Report Coverage
The regulatory information management system market report emphasizes on key regions across the globe to provide better understanding of the product to the users. Also, the report provides market insights into recent developments, trends and analyzes the technologies that are gaining traction around the globe. Furthermore, the report covers in-depth qualitative analysis pertaining to various paradigm shifts associated with the transformation of these solutions.
The report provides detailed analysis of the market while focusing on various key aspects such as competitive analysis, component, deployment, enterprise size, application, end use, and their futuristic growth opportunities.
Regulatory Information Management System Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2024 |
USD 2.11 billion |
|
Revenue forecast in 2032 |
USD 4.35 billion |
|
CAGR |
9.5% from 2024 – 2032 |
|
Base year |
2023 |
|
Historical data |
2019 – 2022 |
|
Forecast period |
2024 – 2032 |
|
Quantitative units |
Revenue in USD billion and CAGR from 2024 to 2032 |
|
Segments covered |
By Component, By Deployment, By Enterprise Size, By Application, By End Use, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America; Middle East & Africa |
|
Customization |
Report customization as per your requirements with respect to countries, region and segmentation. |
FAQ's
The Regulatory Information Management System Market report covering key segments are component, deployment, enterprise size, application, end-use, and region.
Regulatory Information Management System Market Size Worth USD 4.35 Billion by 2032
Regulatory Information Management System Market exhibiting the CAGR of 9.5% during the forecast period.
North America is leading the global market.
The key driving factors in Regulatory Information Management System Market are 1. Increasing Complexity of Regulatory Requirements is driving regulatory information management system market growth.
