
Acute Repetitive Seizures Market Share, Size, Trends, Industry Analysis Report
By Product (USL-261, NRL-1, AZ-002, Diastat Rectal Gel, Others); By End Use; By Region; And Segment Forecasts, 2024 - 2032
- Published Date:Mar-2024
- Pages: 114
- Format: PDF
- Report ID: PM4747
- Base Year: 2023
- Historical Data: 2019-2022
Report Outlook
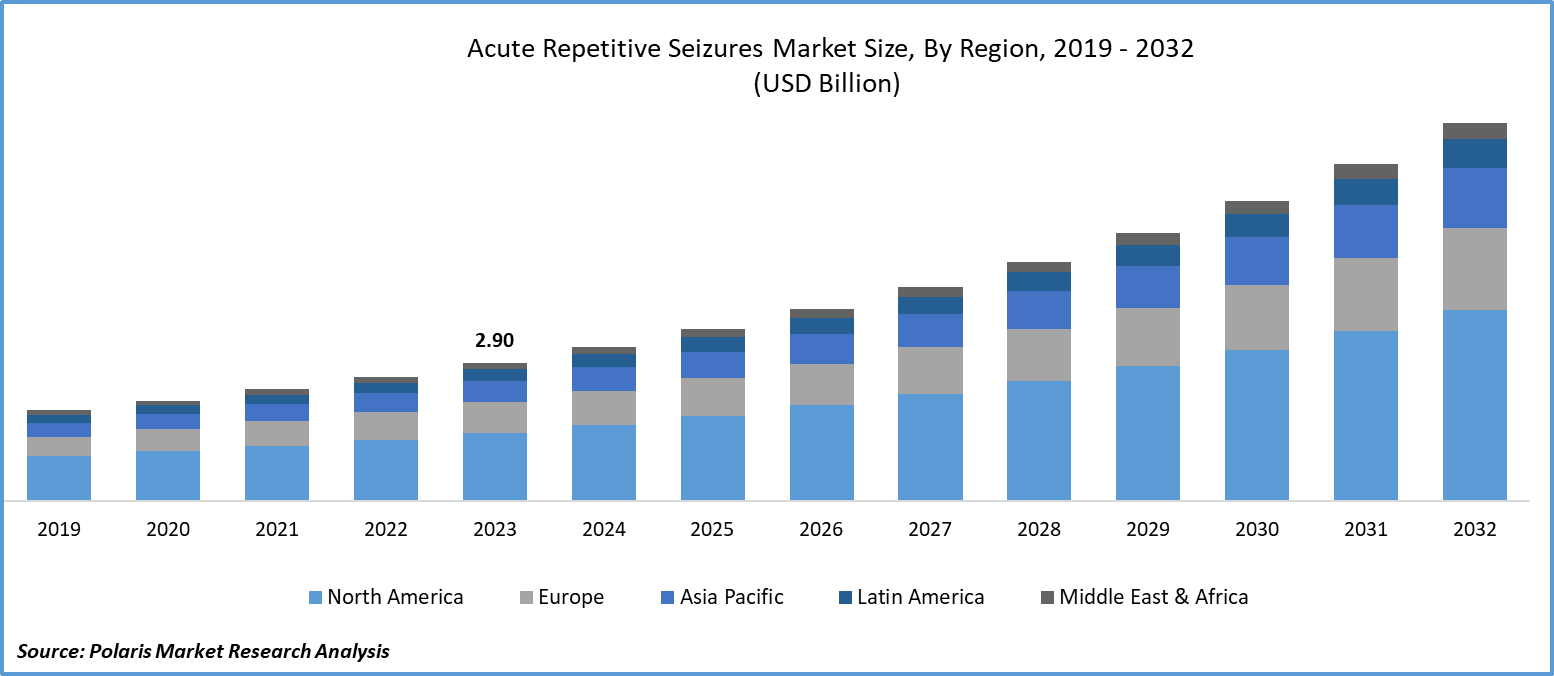
The global acute repetitive seizures market was valued at USD 2.90 billion in 2023 and is expected to grow at a CAGR of 11.9% during the forecast period.
Market growth is being propelled by a rising number of clinical studies, a heightened emphasis on the introduction of new medicines, and an increased demand for nasal sprays as a treatment for epilepsy. Additionally, the surge in regulatory approvals and promising treatments in the pipeline is expected to drive the development of the acute repetitive seizures market throughout the projected period. In addition to efforts to reduce healthcare spending, governments are actively working to minimize hospital stays and on-site treatment expenses through the implementation of outpatient care models, such as home healthcare and clinics.
Acute Repetitive Seizures (ARS), also known as re-current, serial seizures, represent a treatment-resistant epileptic phenomenon characterized by a closely connected series of seizures exceeding a patient's expected baseline seizure incidence. This condition heightens the risk of hospitalization & progression to the status epilepticus, the worst seizure emergency, underscoring the critical need for prompt and effective therapy. In this orphan indication, benzodiazepines are commonly employed for treatment.
To Understand More About this Research: Request a Free Sample Report
Government bodies recommend using cluster seizure treatments as an addition to patients' chronic Antiepileptic Drug (AED) regimen, to be administered on an as-needed basis. Although drug therapy is highly effective for most patients with cluster seizures, various challenges and medical needs remain unmet, including adverse effects, drug-induced seizures, and a shortage of antiepileptic drugs capable of preventing the onset of seizures and associated comorbidities.
Growth is also favored by high unmet clinical needs for both patients and caretakers, growing awareness of epilepsy, an increasing prevalence of neurological disorders, and the availability of advanced product pipelines. According to the World Health Organization (WHO), epilepsy, a neurological condition, affects approximately 50 million people worldwide. While drug therapy proves effective for most patients dealing with cluster seizures, there exist various treatment challenges and unmet medical requirements. These challenges include drug-induced seizures, adverse reactions, and a lack of antiepileptogenic agents capable of preventing the development of seizures and associated comorbidities.
In the Acute Repetitive Seizures (ARS) market, collaboration is the key to progress. Partnerships between medical experts, pharmaceutical companies, and patient advocacy groups drive innovation and address the challenges of this condition. Through shared goals, open communication, and resilience, stakeholders work together to advance scientific understanding, develop new treatments, and improve patient outcomes. Collaboration paints a landscape of hope for those affected by ARS, breaking down barriers and paving the way for a brighter future.
For instance, In January 2022, Neurelis and Aculys Pharma forged an exclusive collaboration to promote and propel the development of VALTOCO in the Asia Pacific region. Based in Japan, Aculys Pharma is dedicated to driving advancements and commercialization in the fields of psychiatry and neurology.
Treatment for acute repetitive seizures typically involves the use of antiepileptic drugs, and in certain cases, surgical procedures may be employed to remove a portion of the brain.

What are the Market Drivers Driving the Demand for?
Rising Prevalence of Neurological Disorders is Projected to Spur the Market Growth
Neurological disorders, especially epilepsy, have been steadily increasing, which has contributed to the growth of the Acute Repetitive Seizures (ARS) market. Epilepsy is a chronic neurological disorder that results in recurring seizures and affects millions of people worldwide. As the number of people with epilepsy continues to rise globally, the demand for effective treatments, including those specifically designed to target ARS, is also increasing.
Absence-related seizures (ARS) is a type of epilepsy that involves clusters of seizures occurring within a short period, often over a span of hours or days. These repetitive and prolonged seizure episodes can severely affect individuals' health, safety, and overall quality of life. Therefore, it is essential to develop specialized treatments to manage ARS and prevent seizure recurrence. The increasing incidence of epilepsy cases worldwide is directly contributing to the rising prevalence of ARS. As more people are diagnosed with epilepsy, a subset of these patients will experience ARS episodes, further driving the demand for ARS treatments. This growing patient population highlights the importance of developing and advancing therapeutic interventions specifically designed to address the unique challenges posed by ARS.
Furthermore, the recognition of ARS as a distinct clinical entity within the epilepsy spectrum has led to greater awareness and understanding among healthcare professionals. This increased awareness facilitates early identification and diagnosis of ARS cases, leading to timely intervention and management strategies.
Which Factor is Restraining the Demand for Acute Repetitive Seizures?
Limited Availability of Specialized Diagnostic Tests or Monitoring Devices
The diagnostic challenge for detecting and recording Acute Repetitive Seizures (ARS) is compounded by the limited availability of specialized diagnostic tests or monitoring devices. While conventional methods like electroencephalography (EEG) and seizure diaries may help diagnose ARS, they may not always accurately capture the complete extent or frequency of seizure clusters.
The diagnostic uncertainty surrounding ARS can have several implications for patient care and clinical management. Delayed or inaccurate diagnosis may result in suboptimal treatment outcomes, increased healthcare utilization, and heightened anxiety for patients and caregivers. Addressing the diagnostic challenges associated with ARS requires concerted efforts from healthcare professionals, researchers, and regulatory bodies. Developing standardized diagnostic criteria, enhancing clinician awareness and education, and investing in innovative diagnostic technologies are essential steps toward improving the accuracy and timeliness of ARS diagnosis. By overcoming these restraints, healthcare providers can better identify and manage ARS cases, ultimately improving patient outcomes and quality of life.
Report Segmentation
The market is primarily segmented based on product, end-use, and region.
|
By Product |
By End Use |
By Region |
|
|
|
To Understand the Scope of this Report: Speak to Analyst
Category Wise Insights
By Product Insights
Based on Product analysis, the market is segmented on the basis of USL-261, NRL-1, AZ-002, diastat rectal gel, and others. The USL-261 segment held the largest share in 2023. USL-261, also known as intranasal midazolam, has demonstrated efficacy and safety in the management of ARS. Clinical studies have shown that intranasal midazolam effectively terminates acute seizure episodes, including those associated with ARS, with a rapid onset of action and minimal adverse effects.
One of the key advantages of USL-261 is its ease of administration. Being an intranasal formulation, it can be quickly and easily administered by caregivers or healthcare professionals, even in non-hospital settings such as homes or schools. This convenience of administration makes USL-261 an attractive option for managing acute seizure clusters, including those characteristics of ARS. The intranasal route of administration eliminates the need for invasive procedures such as intravenous injections, which can be challenging, especially during seizure episodes. The non-invasive nature of intranasal midazolam may improve patient compliance and acceptance, particularly in pediatric and geriatric populations.
By End Use Insights
Based on type analysis, the market has been segmented on the basis of biotechnology, healthcare & pharmaceuticals, and others. The healthcare & pharmaceuticals segment is anticipated to grow at the fastest CAGR during the projected period. The rising incidence and prevalence of neurological disorders, including epilepsy and Acute Repetitive Seizures (ARS), are driving demand for innovative healthcare and pharmaceutical solutions. As the global population ages and lifestyles change, the burden of neurological disorders is expected to increase, fueling the need for effective treatments and management strategies.
Ongoing research and development efforts in the healthcare and pharmaceutical sectors are leading to the introduction of novel treatment modalities for neurological disorders, including ARS. Innovative pharmaceuticals, medical devices, and therapies are being developed to address the unmet medical needs of patients with ARS, driving market growth. Regulatory agencies are increasingly recognizing the importance of addressing neurological disorders and are expediting the approval process for new healthcare and pharmaceutical products targeting ARS. Favorable regulatory policies and market approvals facilitate the commercialization of innovative treatments, contributing to market growth.
Regional Insights
North America
North America held the largest market share in 2023 due to the increasing number of epilepsy cases reported in the region. The total direct and indirect cost of epilepsy in North America is estimated to be around USD 15.5 billion per year. As a result, the region has a significant target population for epilepsy treatment drugs, which will likely drive market growth. To put this in perspective, approximately 1.2% of the U.S. population has active epilepsy, or around 3.4 million Americans who could benefit from treatments in this market.
North America boasts a well-developed healthcare infrastructure with access to advanced medical facilities, specialized healthcare professionals, and advanced diagnostic and treatment technologies. This infrastructure enables timely diagnosis, effective management, and comprehensive care for patients with ARS. The region has a relatively high prevalence of neurological disorders, including epilepsy and ARS. Factors such as an aging population, lifestyle-related risk factors, and improved disease awareness contribute to the growing burden of neurological disorders in North America, driving demand for ARS treatments. North America is at the forefront of medical research and technological innovation. The region's pharmaceutical and biotechnology industries continuously develop novel therapies, medical devices, and diagnostic tools for neurological disorders, including ARS. These advancements contribute to improved patient outcomes and drive market growth.
Asia Pacific
The Asia Pacific region is projected to grow at the fastest CAGR during the forecast period. The increased healthcare expenditure in developing countries primarily drives the region’s growth. There was a significant gap observed in addressing acute repetitive seizures in this region. With over 12 million cases of epilepsy in India and around 10 million cases in China, there is a substantial demand for antiseizure drugs in these developing countries. This demand not only reflects a healthcare need but also signifies a shift in market potential from the comparatively saturated regions to the growing and under-served markets. The increased focus on healthcare infrastructure and rising awareness about epilepsy contribute to the favorable conditions for market growth in these developing nations.
Competitive Landscape
The acute repetitive seizures market is characterized by fragmentation, with competition expected to intensify due to the presence of numerous players. Major service providers within the market continuously enhance their technologies to maintain a competitive edge, prioritizing efficiency, integrity, and safety. They concentrate on forming partnerships, implementing product upgrades, and fostering collaboration to establish a competitive advantage over their counterparts and secure a substantial market share.
Some of the major players operating in the global market include:
- ALEXZA
- Alexza Pharmaceuticals
- Bausch Health Companies Inc.
- Neurelis, Inc.
- Pfizer Inc.
- Sanofi
- UCB S.A. Belgium
- Valeant Pharmaceuticals North America LLC.
- VERITON PHARMA
Recent Developments
-
In January 2022, UCB acquired Midazolam nasal spray (USL-61) from Proximagen, thereby expanding its epilepsy portfolio. This strategic move to acquire USL-261 aligns with UCB's existing and prosperous anti-epilepsy drug portfolio, consolidating the company's position in the global market for epilepsy treatments.
- In October 2021, Alexza commenced a phase 3 trial for Staccato Alprazolam, an acute treatment for epileptic seizures. UCB Pharma is overseeing the trials to evaluate the drug's safety and efficacy.
- In July 2020, Takeda unveiled a global partnership with Ovid Therapeutics to collaborate on the development and commercialization of the Soticlestat. This collaboration aims to bring forward a potentially effective treatment for rare epilepsy syndromes, including specific types of acute recurrent seizures
Report Coverage
The acute repetitive seizures market report emphasizes on key regions across the globe to provide better understanding of the product to the users. Also, the report provides market insights into recent developments, trends and analyzes the technologies that are gaining traction around the globe. Furthermore, the report covers in-depth qualitative analysis pertaining to various paradigm shifts associated with the transformation of these solutions.
The report provides detailed analysis of the market while focusing on various key aspects such as competitive analysis, product, end-users, and their futuristic growth opportunities.
Acute Repetitive Seizures Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2024 |
USD 3.23 billion |
|
Revenue forecast in 2032 |
USD 7.94 billion |
|
CAGR |
11.9% from 2023 – 2032 |
|
Base year |
2023 |
|
Historical data |
2019 – 2022 |
|
Forecast period |
2024 – 2032 |
|
Quantitative units |
Revenue in USD billion and CAGR from 2024 to 2032 |
|
Segments covered |
By Product, By End Use, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
|
Customization |
Report customization as per your requirements with respect to countries, region, and segmentation. |
FAQ's
key companies in Acute Repetitive Seizures Market are ALEXZA, Alexza Pharmaceuticals, Bausch Health Companies Inc., Neurelis, Inc., Pfizer Inc., Sanofi, UCB S.A. Belgium
The global acute repetitive seizures market is expected to grow at a CAGR of 11.9% during the forecast period.
The Acute Repetitive Seizures Market report covering key segments are product, end-use, and region.
key driving factors in Acute Repetitive Seizures Market are rising Prevalence of Neurological Disorders
The global acute repetitive seizures market size is expected to reach USD 7.94 billion by 2032
