
U.S. Blood-Based Biomarker for Parkinson’s Disease Market Size, Share, Industry Analysis Report
By Biomarker Type [Alpha-Synuclein (α-syn), Neurofilament Light Chain (Nfl)], By Application, By Technology Platform, By End User – Market Forecast, 2025–2034
- Published Date:Jul-2025
- Pages: 128
- Format: PDF
- Report ID: PM5993
- Base Year: 2023
- Historical Data: 2020-2023
Overview
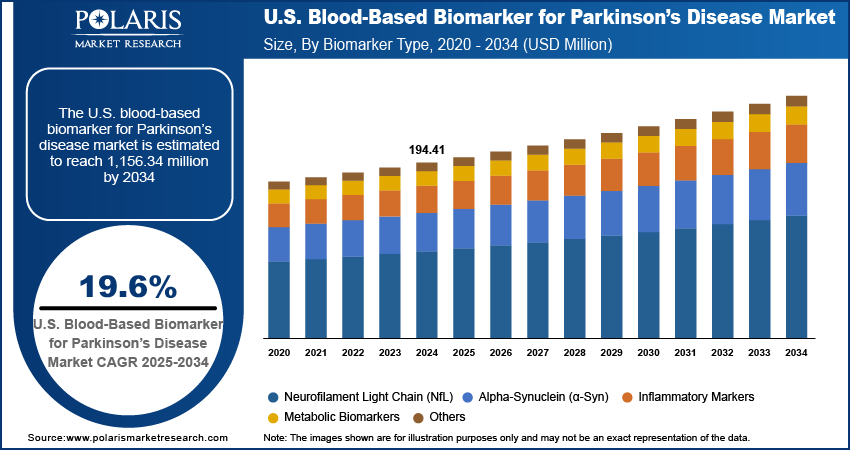
The U.S. blood-based biomarker for Parkinson’s disease market size was valued at USD 194.41 million in 2024, growing at a CAGR of 19.6% from 2025 to 2034. Key factors driving demand for blood-based biomarkers for Parkinson’s disease include the expansion of clinical trials and research initiatives, rising prevalence of Parkinson’s disease, increasing shift toward personalized medicine biomarkers, and advanced disease monitoring.
Key Insights
- The neurofilament light chain (nfl) segment is expected to witness the highest CAGR of 21.00% during the forecast period.
- The diagnostic biomarkers dominated the revenue share and was valued at USD 81.60 million in 2024.
- The αSyn-SAA segment accounted for a significant share of the market in 2024.
- The pharmaceutical companies segment is expected to witness substantial growth during the forecast period.
Blood-based biomarkers for Parkinson’s disease are measurable biological indicators found in blood that aid in detecting, monitoring, and understanding the progression of the disease. The growing movement toward personalized medicine and advanced disease monitoring is boosting the growth opportunities. There is an increasing demand for minimally invasive tools that provide personalized insights into disease onset, progression, and treatment response, as precision medicine gains momentum. Blood-based biomarkers offer a promising approach for early detection and ongoing monitoring, eliminating the need for more invasive or expensive diagnostic methods. It also aligns with the healthcare industry’s shift toward patient-specific treatment strategies.
The expansion of research initiatives and clinical trials focused on neurodegenerative disorders further enhances opportunities for industry across the U.S. Public and private sector institutions are investing in the identification and validation of reliable blood-based biomarkers, leading to an increase in collaborative studies and longitudinal trials. For instance, in July 2024, the FNIH launched the AMP PDRD initiative, a public-private partnership worth USD 21 million that involves the NIH, FDA, and The Michael J. Fox Foundation. The project's goal is to improve the differential diagnosis of Parkinson's disease and related disorders, enabling earlier intervention. These initiatives aim to establish standardized biomarker panels for clinical practice, thereby enhancing diagnostic accuracy and facilitating the development of targeted therapies. Additionally, the continuous evolution of omics technologies and bioinformatics platforms further supports this expansion, creating a robust pipeline of biomarkers within academic and clinical research environments.
Industry Dynamics
- The increasing cases of Parkinson’s disease are boosting the demand for advanced diagnostic methods, with blood-based biomarkers emerging as a promising option for earlier and more accurate detection.
- Advancements in biomarker science, especially in discovery platforms and clinical validation, are enhancing the reliability and utility of blood-based tests.
- Optimized blood-based diagnostic tests can facilitate large-scale early detection of Parkinson’s disease, enabling timely interventions to slow its progression.
- Many blood-based biomarkers overlap with other neurodegenerative conditions, hindering Parkinson’s-specific accuracy and clinical use.
Rising Prevalence of Parkinson’s Disease: The demand for blood-based biomarkers is increasing due to the rising prevalence of Parkinson’s disease in the U.S., which boosts the need for efficient and scalable diagnostic and monitoring solutions. The healthcare system is under pressure to adopt tools that can support earlier diagnosis, track disease progression, and tailor treatment approaches, with a growing aging population, among whom Parkinson’s disease is most common. In February 2025, NINDS reported 500,000 diagnosed Parkinson's cases in the U.S., and an estimation suggests the actual number may be closer to 1 million due to underdiagnoses. Blood-based biomarkers provide a noninvasive and accessible solution, enabling clinicians to manage increasing patient volumes while enhancing diagnostic precision. This rising disease burden underscores the clinical relevance of such biomarkers and drives investments in their development and integration into routine care pathways.
Advancements in Biomarker Discovery and Validation: The landscape of potential diagnostic and prognostic tools is expanding due to the advancements in biomarker discovery and validation, which are driving the market forward. Progress in genomics, proteomics, and metabolomics technologies has enabled the identification of novel blood-based biomarker candidates associated with Parkinson’s disease pathology. According to a December 2023 report, Duke University researchers developed "Mito DNADX", an NIH-funded blood test that detects Parkinson-related mitochondrial DNA damage. The test accurately identified patients across genetic and medication variations, offering potential for improved diagnosis and treatment tracking. These innovations, associated with improvements in assay sensitivity and standardization, are enabling the reliable detection of disease-specific biological signatures in peripheral blood. Therefore, as validation protocols become more robust and regulatory pathways become more defined, these advancements facilitate the translation of research findings into clinically applicable tools, thereby strengthening their adoption across research and healthcare environments.
Segmental Insights
Biomarker Type Analysis
Based on biomarker type, the U.S. blood-based biomarker for Parkinson’s disease market segmentation includes alpha-synuclein (α-syn), neurofilament light chain (NfL), inflammatory markers, metabolic biomarkers, and others. The neurofilament light chain (nfl) segment is expected to witness the highest CAGR of 21.00% during the forecast period owing to its emerging role as a reliable and sensitive marker of neurodegeneration. Elevated NfL levels in the bloodstream are strongly linked with axonal injury and disease advancement in Parkinson’s patients, positioning it as a valuable tool for both early diagnosis and disease monitoring. Its ability to indicate neuronal damage across various stages of Parkinson’s improves its application in clinical research and therapeutic trials. Additionally, the development of advanced assay platforms is enhancing measurement accuracy and consistency, thereby refining the clinical integration of NfL biomarkers.
Application Analysis
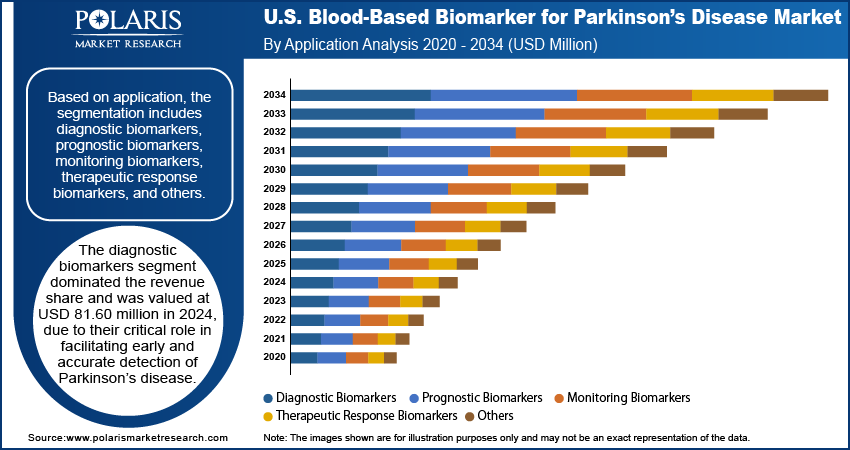
In terms of application, the U.S. blood-based biomarker for Parkinson’s disease market is segmented into diagnostic biomarkers, prognostic biomarkers, monitoring biomarkers, therapeutic response biomarkers, and others. The diagnostic biomarkers segment dominated the revenue share and was valued at USD 81.60 million in 2024, primarily due to their critical role in facilitating early and accurate detection of Parkinson’s disease. Their effectiveness is particularly evident during the prodromal and early symptomatic phases, where timely diagnosis can significantly impact treatment strategies. The growing preference for noninvasive methods is driving the shift toward blood-based diagnostics, offering a more accessible and scalable alternative to traditional imaging or cerebrospinal fluid analyses. Moreover, enhanced clinical guidelines and rising awareness about the benefits of early detection are also supporting the widespread adoption of diagnostic biomarkers in varied healthcare environments.
Technology Platform Analysis
The U.S. blood-based biomarker for Parkinson’s disease market, based on technology platform, is segmented into alpha-synuclein seeding amplification assay (αsyn-saa), ELISA-based assays, immunoassays, mass spectrometry, and others. The αSyn-SAA segment accounted for a significant share in 2024, supported by its high sensitivity and specificity in identifying misfolded alpha-synuclein aggregates. This assay technology represents an advancement in Parkinson’s diagnostics, enabling the detection of pathological markers in blood samples with enhanced accuracy. Its ability to identify disease presence even before the manifestation of clinical symptoms makes it particularly valuable for early-stage detection. Furthermore, the assay’s effectiveness in differentiating patient groups and its growing validation in real-world clinical environments are encouraging its use across diagnostic workflows.
End User Analysis
The U.S. blood-based biomarker for Parkinson’s disease market segmentation, based on end user, includes hospitals and clinics, diagnostic laboratories, research institutes, and pharmaceutical companies. The pharmaceutical companies segment is expected to witness substantial growth during the forecast period, driven by their increasing investments in developing targeted, disease-modifying treatments for Parkinson’s disease. Blood-based biomarkers are becoming increasingly essential tools in drug development pipelines, aiding in patient stratification, monitoring therapeutic responses, and enhancing the efficiency of clinical trials. Their use in early-phase trials is enabling more precise assessments of drug efficacy and safety, thereby accelerating development timelines. Collaborations among pharmaceutical firms, academic institutions, and diagnostic technology developers are further expanding the use of biomarkers across the pharmaceutical landscape.
Key Players and Competitive Analysis
The U.S. blood-based biomarker for Parkinson’s disease market is witnessing significant revenue growth, driven by emerging technologies and strategic investments in research and development (R&D). Major players are leveraging competitive intelligence and strategy to capitalize on latent demand and opportunities, particularly in developed markets. Industry trends highlight a shift toward noninvasive diagnostics, creating expansion opportunities for small and medium-sized businesses. Technological advancements are accelerating biomarker discovery, while economic and geopolitical shifts influence region-wise market size and adoption. Expert's insight suggests that sustainable value chains and future development strategies will be critical to maintaining a competitive positioning. Companies are also focusing on revenue growth analysis to identify high growth markets, ensuring alignment with macroeconomic trends and business transformation initiatives. This dynamic landscape underscores the importance of vendor strategies and growth projections to navigate supply chain disruptions and unlock revenue opportunities.
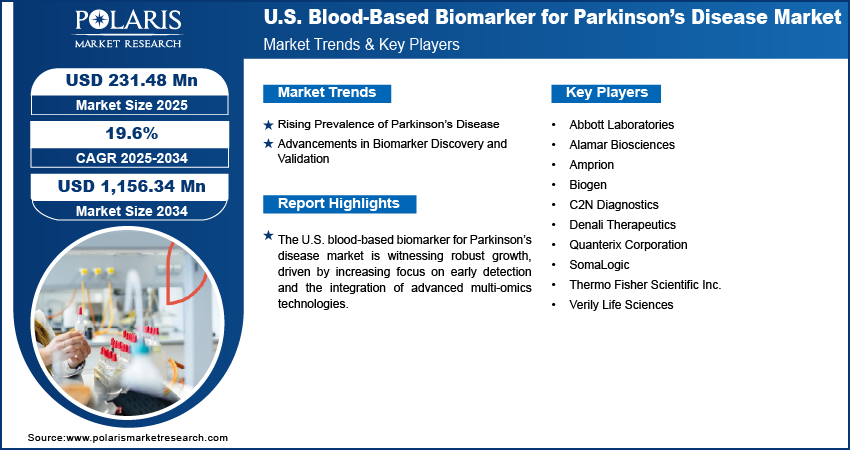
Major companies operating in the U.S. blood-based biomarker for Parkinson’s disease market include Abbott Laboratories, Alamar Biosciences, Amprion, Biogen, C2N Diagnostics, Denali Therapeutics, Quanterix Corporation, SomaLogic, Thermo Fisher Scientific Inc., and Verily Life Sciences.
Key Players
- Abbott Laboratories
- Alamar Biosciences
- Amprion
- Biogen
- C2N Diagnostics
- Denali Therapeutics
- Quanterix Corporation
- SomaLogic
- Thermo Fisher Scientific Inc.
- Verily Life Sciences
U.S. Blood-Based Biomarker for Parkinson’s Disease Industry Developments
- October 2024: ADx NeuroSciences and Alamar Biosciences partnered to develop customized biomarker assays using Alamar's NULISA platform. The collaboration aims to enhance the development of therapeutic treatment for neurological diseases, focusing on Alzheimer’s, Parkinson’s, and ALS biomarkers for pharmaceutical applications.
- January 2025, C2N Diagnostics partnered with The Michael J. Fox Foundation to study links between Alzheimer’s disease and neuronal α-synuclein disorders such as Parkinson’s, Lewy body dementia, and REM behavior disorder, advancing research on shared neurodegenerative mechanisms.
U.S. Blood-Based Biomarker for Parkinson’s Disease Market Segmentation
By Biomarker Type Outlook (Revenue, USD Million, 2020–2034)
- Alpha-Synuclein (α-Syn)
- Neurofilament Light Chain (NfL)
- Inflammatory Markers
- Metabolic Biomarkers
- Others
By Application Outlook (Revenue, USD Million, 2020–2034)
- Diagnostic Biomarkers
- Prognostic Biomarkers
- Monitoring Biomarkers
- Therapeutic Response Biomarkers
- Others
By Technology Platform Outlook (Revenue, USD Million, 2020–2034)
- Alpha-Synuclein Seeding Amplification Assay (αSyn-SAA)
- ELISA-Based Assays
- Immunoassays
- Mass Spectrometry
- Others
By End User Outlook (Revenue, USD Million, 2020–2034)
- Hospitals and Clinics
- Diagnostic Laboratories
- Research Institutes
- Pharmaceutical Companies
U.S. Blood-Based Biomarker for Parkinson’s Disease Market Report Scope
|
Report Attributes |
Details |
|
Market Size in 2024 |
USD 194.41 Million |
|
Market Size in 2025 |
USD 231.48 Million |
|
Revenue Forecast by 2034 |
USD 1,156.34 Million |
|
CAGR |
19.6% from 2025 to 2034 |
|
Base Year |
2024 |
|
Historical Data |
2020–2023 |
|
Forecast Period |
2025–2034 |
|
Quantitative Units |
Revenue in USD Million and CAGR from 2025 to 2034 |
|
Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, and Industry Trends |
|
Segments Covered |
|
|
Regional Scope |
|
|
Competitive Landscape |
|
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, regions, and segmentation. |
FAQ's
The market size was valued at USD 194.41 million in 2024 and is projected to grow to USD 1,156.34 million by 2034.
The market is projected to register a CAGR of 19.6% during the forecast period.
A few of the key players in the U.S. market are Abbott Laboratories, Alamar Biosciences, Amprion, Biogen, C2N Diagnostics, Denali Therapeutics, Quanterix Corporation, SomaLogic, Thermo Fisher Scientific Inc., and Verily Life Sciences.
The diagnostic biomarkers segment dominated the revenue share and was valued at USD 81.60 million in 2024.
The neurofilament light chain (nfl) segment is expected to witness the highest CAGR of 19.50% during the forecast period.
