
Blood-Based Biomarker for Parkinson’s Disease Market Size, Share, Industry Analysis Report
By Biomarker Type [Alpha-Synuclein (α-Syn), Neurofilament Light Chain (Nfl)], By Application, By Technology Platform, By End User, By Region – Market Forecast, 2025–2034
- Published Date:Jul-2025
- Pages: 129
- Format: PDF
- Report ID: PM5992
- Base Year: 2024
- Historical Data: 2020-2023
Overview
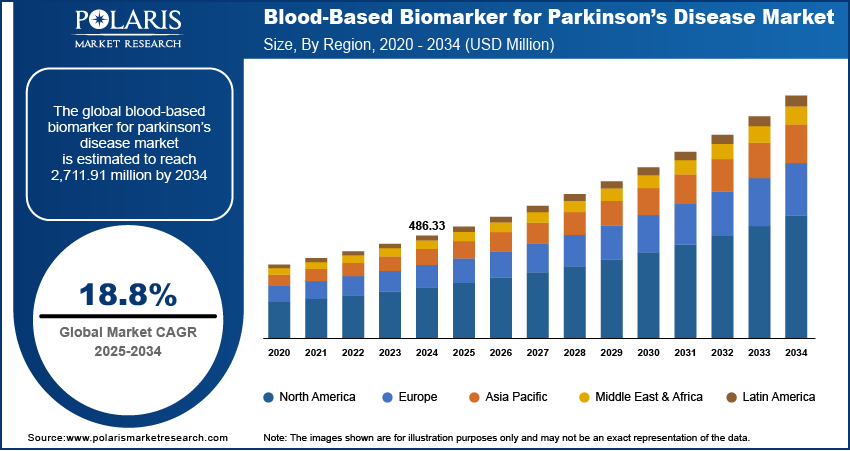
The global blood-based biomarker for parkinson’s disease market size was valued at USD 486.33 million in 2024, growing at a CAGR of 18.8% from 2025–2034. Key factors driving demand for blood-based biomarker for parkinson’s disease include the expansion of clinical trials and research initiatives, rising prevalence of parkinson’s disease, and advancements in biomarker discovery and validation.
Key Insights
- The neurofilament light chain (nfl) segment is expected to witness the highest CAGR of 19.00% during the forecast period.
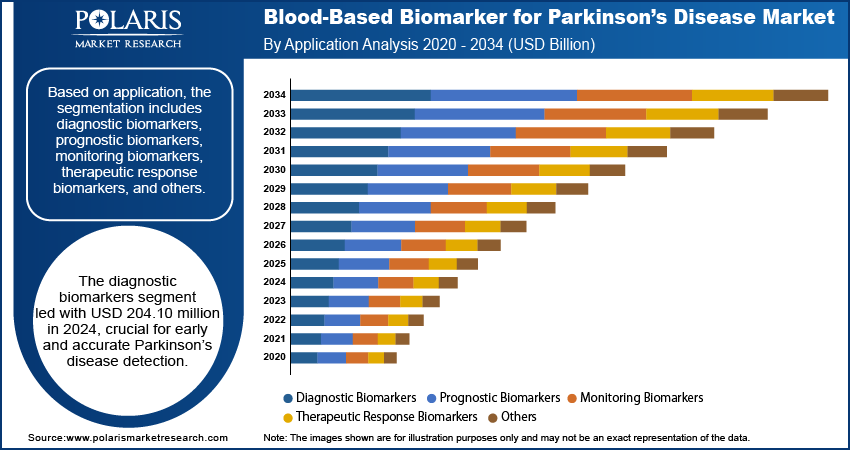
- The diagnostic biomarkers segment dominated the revenue share and was valued at USD 204.10 million in 2024.
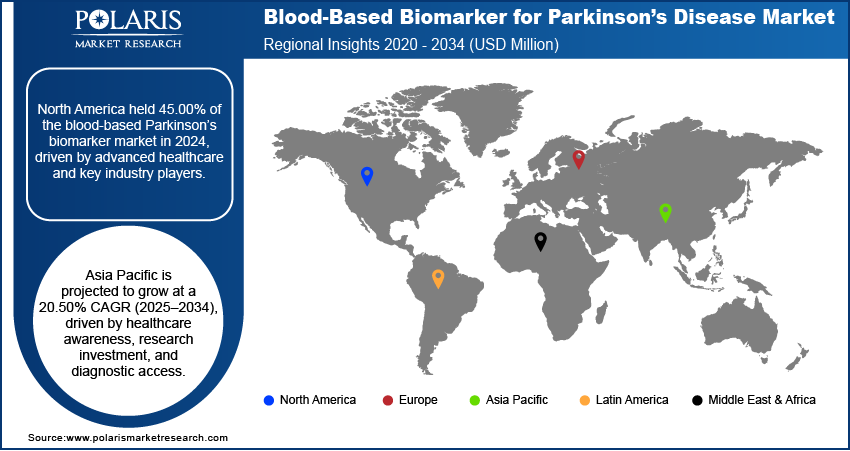
- North America accounted for a 45.00% share of the global blood-based biomarker for Parkinson’s disease market in 2024.
- The U.S. held a 19.30% market share in North America blood-based biomarker for Parkinson’s disease landscape in 2024.
- The market in Asia Pacific is projected to register a CAGR of 20.50% from 2025 to 2034.
- The market in India is expanding due to growing investments in neurology-focused healthcare infrastructure and increasing awareness of the importance of early diagnosis.
Blood-based biomarkers for parkinson’s disease refer to measurable biological molecules found in blood that aid in the early diagnosis, monitoring of disease progression, and assessment of treatment response in parkinson’s disease. The market for these biomarkers is gaining momentum, driven by the expansion of clinical trials and research initiatives aimed at identifying specific biomarkers that differentiate parkinson’s disease from other neurodegenerative disorders. Researchers are increasingly focusing on blood as a noninvasive and scalable medium for identifying diagnostic indicators, as the understanding of the disease’s molecular and pathological mechanisms improves. This has led to the integration of advanced technologies, such as proteomics and genomics, into clinical studies, further advancing the biomarker discovery process and contributing to the overall growth.
The market is further driven by increased funding and growing investments from both public and private sectors, fostering innovation and commercialization of blood-based biomarker solutions. In July 2024, the FNIH launched the AMP PDRD initiative, a USD 21 million public-private partnership involving NIH, FDA, and The Michael J. Fox Foundation. The project aims to enhance the differential diagnosis of Parkinson's disease and related disorders, facilitating earlier intervention. Biopharmaceutical companies and diagnostic developers are collaborating with academic and clinical institutions to accelerate the development of their product pipelines. Financial support is also encouraging start-ups and established players to explore novel biomarker candidates and validate them using innovative tools. This growing financial and strategic commitment is enabling a more robust infrastructure for Parkinson’s disease diagnostics, supporting the scaling and accessibility of blood-based biomarker tests and positioning them as a critical tool in personalized neurology.
Industry Dynamics
- The increasing global prevalence of parkinson's disease is creating demand for improved diagnostic solutions, particularly blood-based biomarkers that enable earlier and more precise detection compared to traditional methods.
- Progress in biomarker research, such as novel discovery platforms and rigorous clinical validation protocols, is enhancing the diagnostic accuracy and practical applicability of blood tests.
- Blood tests enable early disease detection and treatment, shifting from reactive to preventive care and reducing healthcare burdens.
- Many blood-based biomarkers show overlap with other neurological disorders, making an accurate parkinson's diagnosis difficult. This reduces clinical adoption despite technological advances.
Rising Prevalence of Parkinson’s Disease: The rising prevalence of parkinson’s disease is driving the growth opportunities, as it increases the demand for early and accurate diagnostic tools. Healthcare systems are under pressure to adopt scalable and noninvasive diagnostic methods that facilitate timely intervention and disease management, as the number of individuals affected by the condition continues to grow. A February 2025 National Institute of Neurological Disorders and Stroke report estimated 500,000 diagnosed Parkinson's cases in the U.S., with actual prevalence potentially reaching 1 million due to underdiagnoses. The progressive nature of PD also impacts families and caregivers nationwide. Blood-based biomarkers offer a practical solution by enabling accessible, cost-effective, and repeatable testing compared to more invasive procedures, such as cerebrospinal fluid analysis or neuroimaging. Therefore, as the patient population expands, the need to distinguish Parkinson’s from similar neurodegenerative diseases further highlights the role of reliable blood-based diagnostics, thereby boosting expansion opportunities.
Advancements in Biomarker Discovery and Validation: Advancements in biomarker discovery and validation are also instrumental in driving the expansion opportunities, as they enhance the specificity, sensitivity, and clinical utility of blood-based diagnostics. In December 2023, Researchers at Duke University, funded by the NIH, developed a blood test called "Mito DNADX" that detects mitochondrial DNA damage associated with parkinson's disease. The test identified Parkinson's patients regardless of genetic status or medication use, potentially aiding diagnosis and treatment monitoring. Advanced technologies, such as high-throughput screening, proteomics, and machine learning algorithms, are enabling researchers to identify novel biomarkers with increased diagnostic accuracy. Additionally, improved validation protocols are ensuring that these biomarkers are clinically relevant and reproducible across diverse patient populations. As a result, these technological and methodological breakthroughs are accelerating the transition of blood-based biomarkers from research to routine clinical practice, thereby reinforcing their adoption in the diagnosis and monitoring of Parkinson's disease.
Segmental Insights
Biomarker Type Analysis
Based on biomarker type, the segmentation includes alpha-synuclein (α-syn), neurofilament light chain (NfL), inflammatory markers, metabolic biomarkers, and others. The neurofilament light chain (nfl) segment is expected to witness the highest CAGR of 19.00% during the forecast period due to its growing relevance as a sensitive and reliable indicator of neurodegeneration. NfL levels in blood have shown a strong correlation with axonal damage and disease progression in parkinson’s patients, making them highly valuable for early detection and monitoring. The ability of NfL to reflect neuronal injury across multiple stages of the disease has increased its utility in clinical trials and research environments. Furthermore, the adoption of advanced assay technologies is enhancing the precision and reproducibility of NfL measurements, thus accelerating its integration into diagnostic and prognostic workflows.
Application Analysis
In terms of application, the segmentation includes diagnostic biomarkers, prognostic biomarkers, monitoring biomarkers, therapeutic response biomarkers, and others. The diagnostic biomarkers segment dominated the revenue share and was valued at USD 204.10 million in 2024. Diagnostic biomarkers play a pivotal role in the early and accurate detection of parkinson's, especially in the prodromal and early symptomatic stages. The increasing demand for noninvasive diagnostic tools has led to a shift toward blood-based biomarkers, offering a less complex and more scalable alternative to imaging or cerebrospinal fluid-based tests. Diagnostic biomarkers facilitate quicker clinical decision-making and can be deployed across diverse healthcare environments. Their adoption is further supported by growing awareness and improved clinical guidelines that highlight the importance of early diagnosis to initiate timely therapeutic interventions.
Technology Platform Analysis
Based on technology platform, the segmentation includes alpha-synuclein seeding amplification assay (αsyn-saa), ELISA-based assays, immunoassays, mass spectrometry, and others. The αSyn-SAA segment accounted for significant share of the market in 2024, driven by its ability to detect misfolded alpha-synuclein aggregates with high sensitivity and specificity. This assay technology has emerged as a critical advancement in parkinson’s diagnostics, particularly for identifying the pathological hallmark of the disease in blood samples. αSyn-SAA has enabled improved grouping of patient populations and supports early detection, even before clinical symptoms fully display. Moreover, the robust performance and growing validation of this assay in clinical settings are contributing to its wider adoption and ongoing integration into diagnostic pipelines.
End User Analysis
The segmentation, based on end user, includes hospitals and clinics, diagnostic laboratories, research institutes, and pharmaceutical companies. The pharmaceutical companies segment is expected to witness fastest growth during the forecast period attributed to their increasing focus on developing disease-modifying therapies and personalized treatments. Blood-based biomarkers are crucial tools in drug development, enabling patient stratification, monitoring treatment efficacy, and reducing trial timelines. The integration of biomarkers into early-phase clinical trials is improving the precision and outcomes of parkinson’s drug development programs. This, combined with strategic partnerships with research institutions and diagnostic developers, is expanding the use of blood-based biomarkers in the pharmaceutical sector.
Regional Analysis
The North America blood-based biomarker for parkinson’s disease market accounted for 45.00% of the global market share in 2024. This dominance is attributed to its advanced healthcare infrastructure and the strong presence of major industry players. The region benefits from substantial investments in neurological research and early adoption of innovative diagnostic tools. In October 2024, the University of Miami committed USD 30 million over five years to enhance neuroscience and aging research. The funding will establish a computational biology program and foster cross-disciplinary collaboration across multiple institutes to address complex health challenges. Regulatory support and robust funding mechanisms are facilitating the rapid validation and commercialization of biomarkers. Additionally, the region's focus on precision medicine and increasing prevalence of Parkinson’s disease are accelerating the demand for noninvasive blood-based diagnostic solutions.
U.S. Blood-Based Biomarker for Parkinson’s Disease Market Insight
The U.S. held a 19.30% share of the North America market in 2024, driven by its strong network of clinical research institutions and high rate of early adoption of advanced diagnostic technologies. Federal support for neurological research and widespread participation in parkinson 's-related clinical trials are accelerating the integration of blood-based biomarkers into routine diagnostics. Additionally, collaborations between biotech firms and healthcare providers are enhancing the commercial availability and clinical utility of these biomarker tests nationwide.
Asia Pacific Blood-Based Biomarker for Parkinson’s Disease Market Trends
The market in Asia Pacific is projected to register a CAGR of 20.50% from 2025 to 2034, driven by rising healthcare awareness, increasing investments in biomedical research, and expanding access to diagnostic services. Countries across the region are prioritizing the management of neurodegenerative diseases, encouraging both the public and private sectors to explore innovative diagnostic technologies. Rapid development of healthcare infrastructure and growth in clinical research collaborations are creating a favorable environment for the adoption of blood-based biomarkers. Moreover, the large and aging population base in the region further amplifies the need for scalable and cost-effective diagnostic tools.
India Blood-Based Biomarker for Parkinson’s Disease Market Overview
The market in India is expanding due to growing investments in neurology-focused healthcare infrastructure and increasing awareness of the importance of early diagnosis of parkinson’s disease. Increasing participation in international research collaborations and the emergence of local diagnostic startups are further strengthening the country’s position in this space. The rising demand for cost-effective, noninvasive diagnostic solutions is also encouraging the adoption of blood-based biomarkers within both urban and emerging tier-2 healthcare environments.
Europe Blood-Based Biomarker for Parkinson’s Disease Market
The blood-based biomarker for parkinson’s disease landscape in Europe is projected to hold a substantial share in 2034 due to the region’s strong focus on translational research and early diagnosis of neurodegenerative diseases. Supportive healthcare policies, well-established academic networks, and cross-border collaborative initiatives are promoting the integration of biomarkers into clinical practice. The presence of leading research institutions and diagnostic firms is accelerating the development and validation of biomarkers. Furthermore, Europe's focus on personalized healthcare and regulatory harmonization is facilitating smoother adoption of advanced blood-based biomarker assays across the region.
UK Blood-Based Biomarker for Parkinson’s Disease Market Assessment
A strong focus on translational research and government-supported initiatives focused on neurodegenerative disorders drive the market growth. The presence of established academic institutions and integrated healthcare systems supports clinical validation and implementation of biomarker-based diagnostics. Additionally, national efforts to adopt precision medicine practices are promoting the incorporation of blood-based biomarker technologies into standard clinical pathways for managing Parkinson’s disease.
Key Players and Competitive Analysis
The field of blood-based biomarker for parkinson’s disease is witnessing intense competition as industry leaders and emerging players leverage strategic investments and technological advancements to capitalize on revenue opportunities. Major players such as Quanterix, Roche, and Abbott dominate developed markets through sustainable value chains and high-precision detection platforms, while startups target emerging markets with cost-effective solutions. Disruptions and trends in ultrasensitive immunoassays and AI-driven biomarker panels are reshaping diagnostics, creating latent demand and opportunities for early detection and therapeutic monitoring. Future development strategies relies on collaborations to address economic and geopolitical shifts in healthcare access. Regional dynamics show North America as leading region in biomarker adoption due to robust R&D infrastructure, while Asia Pacific accelerates growth via expansion opportunities in aging populations. Competitive intelligence highlights divergent approaches: large firms focus on product offerings like α-synuclein assays, whereas small and medium-sized businesses innovate in niche segments. Industry trends highlights the need for business transformation toward decentralized testing and regulatory-compliant platforms.
A few major companies operating in the blood-based biomarker for parkinson’s disease industry include Abbott Laboratories, Adx Neurosciences, Alamar Biosciences, betaSENSE, F. Hoffmann-La Roche Ltd, Merck KGaA, Proteome Sciences, QIAGEN N.V., Quanterix Corporation, and Thermo Fisher Scientific Inc.
Key Players
- Abbott Laboratories
- Adx Neurosciences
- Alamar Biosciences
- betaSENSE
- F. Hoffmann-La Roche Ltd
- Merck KGaA
- Proteome Sciences
- QIAGEN N.V.
- Quanterix Corporation
- Thermo Fisher Scientific Inc.
Industry Developments
- October 2024: ADx NeuroSciences and Alamar Biosciences partnered to develop customized biomarker assays using Alamar's NULISA platform. The collaboration aims to enhance neurological disease therapeutic development, focusing on Alzheimer’s, Parkinson’s, and ALS biomarkers for pharmaceutical applications.
- October 2024: Sunbird Bio presented data at CTAD demonstrating its blood-based α-synuclein biomarker technology can detect brain protein aggregation via blood tests. The method shows high accuracy for diagnosing Parkinson's and other neurodegenerative diseases.
Blood-Based Biomarker for Parkinson’s Disease Market Segmentation
By Biomarker Type Outlook (Revenue, USD Million, 2020–2034)
- Alpha-Synuclein (α-Syn)
- Neurofilament Light Chain (NfL)
- Inflammatory Markers
- Metabolic Biomarkers
- Others
By Application Outlook (Revenue, USD Million, 2020–2034)
- Diagnostic Biomarkers
- Prognostic Biomarkers
- Monitoring Biomarkers
- Therapeutic Response Biomarkers
- Others
By Technology Platform Outlook (Revenue, USD Million, 2020–2034)
- Alpha-Synuclein Seeding Amplification Assay (αSyn-SAA)
- ELISA-Based Assays
- Immunoassays
- Mass Spectrometry
- Others
By End User Outlook (Revenue, USD Million, 2020–2034)
- Hospitals and Clinics
- Diagnostic Laboratories
- Research Institutes
- Pharmaceutical Companies
By Regional Outlook (Revenue, USD Million, 2020–2034)
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Netherlands
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Malaysia
- South Korea
- Indonesia
- Australia
- Vietnam
- Rest of Asia Pacific
- Middle East & Africa
- Saudi Arabia
- UAE
- Israel
- South Africa
- Rest of Middle East & Africa
- Latin America
- Mexico
- Brazil
- Argentina
- Rest of Latin America
Blood-Based Biomarker for Parkinson’s Disease Market Report Scope
|
Report Attributes |
Details |
|
Market Size in 2024 |
USD 486.33 Million |
|
Market Size in 2025 |
USD 575.43 Million |
|
Revenue Forecast by 2034 |
USD 2,711.91 Million |
|
CAGR |
18.8% from 2025 to 2034 |
|
Base Year |
2024 |
|
Historical Data |
2020–2023 |
|
Forecast Period |
2025–2034 |
|
Quantitative Units |
Revenue in USD Million and CAGR from 2025 to 2034 |
|
Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, and Industry Trends |
|
Segments Covered |
|
|
Regional Scope |
|
|
Competitive Landscape |
|
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, regions, and segmentation. |
FAQ's
The global market size was valued at USD 486.33 million in 2024 and is projected to grow to USD 2,711.91 million by 2034.
The global market is projected to register a CAGR of 18.8% during the forecast period.
North America accounted for 45.00% of global market share in 2024.
A few of the key players in the market are Abbott Laboratories, Adx Neurosciences, Alamar Biosciences, betaSENSE, F. Hoffmann-La Roche Ltd, Merck KGaA, Proteome Sciences, QIAGEN N.V., Quanterix Corporation, and Thermo Fisher Scientific Inc.
The diagnostic biomarkers segment dominated the revenue share and was valued at USD 204.10 million in 2024.
The neurofilament light chain (NfL) segment is expected to witness the fastest growth with a CAGR of 19.00% during the forecast period.
