
Electronic Clinical Outcome Assessment Solutions Market Share, Size, Trends, Industry Analysis Report
By Delivery Mode (On-premise, Web & Cloud-based); By End-Use; By Region; Segment Forecast, 2024 - 2032
- Published Date:Jan-2024
- Pages: 118
- Format: pdf
- Report ID: PM3025
- Base Year: 2023
- Historical Data: 2019-2022
Report Outlook
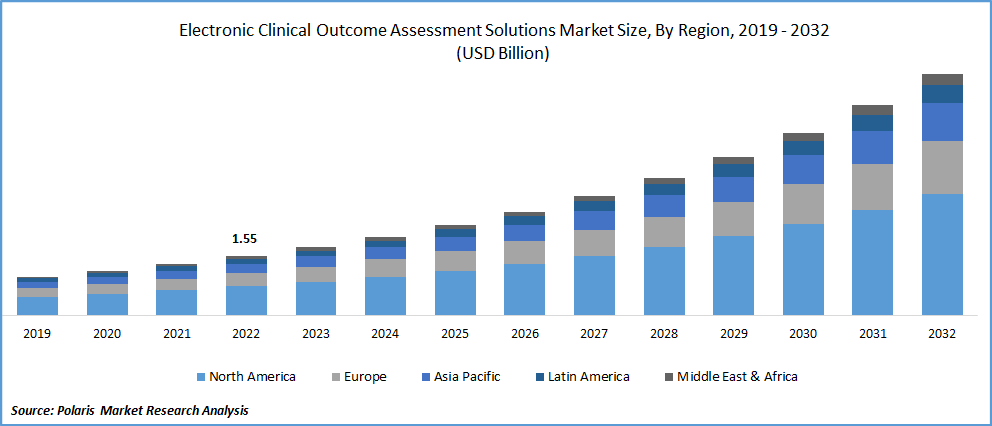
The global electronic clinical outcome assessment solutions market was valued at USD 1.78 billion in 2023 and is expected to grow at a CAGR of 15.0% during the forecast period.
The market is expected to expand as a result of factors such as mounting pressure on pharmaceutical firms to lower overall costs for new drug development processes, which has led to a move away from paper-based processes and toward computerized data gathering. Data collecting with electronic clinical outcome assessment systems improve the caliber of the information gathered combines the data collection procedures and offers consumers significant advantages including data analysis.
Know more about this report: Request for sample pages
Furthermore, the need for innovation to produce novel treatments and medications is growing as a result of the healthcare industry's rapid development. Due to the increase in research studies, a uniform data collection mechanism is needed. Therefore, the demand for the product throughout the predicted period would be affected by all of the aforementioned factors. For instance, the new version of assisTek's electronic clinical outcome assessment (eCOA) platform, which is entirely cloud-based and has improvements for patients, clinical teams, sponsors, and contract research companies, was released in June 2021.
Globally, communities, businesses, and people are being negatively impacted by the ongoing COVID-19 pandemic, which is a rare cause for alarm. A significant rise in healthcare costs as well as the immediate and direct potential effects of COVID-19 have already resulted in the death of millions of people. Medical monitoring and safety reporting are crucial because various potential treatments were explored before the introduction of vaccines and used to treat a coronavirus-induced infection. As a result, there has been an increase in demand for electronic clinical outcome assessment solutions market, and more growth is anticipated soon.
The Habibesadat Shakeri et, al research article released in August 2021, worked on the link between serum levels of zinc, vit B12 & D, and the clinical outcomes in patients suffering from COVID-19. They gathered demographic information, clinical traits, and serum biochemical parameter values during the 1st week, & clinical outcomes from the EMRs. The findings demonstrate that increased hospital stay for COVID-19 patients was unaffected by the serum concentrations of zinc, vitamin B12, & D during admission. Clinical results in COVID-19 patients appear to be generally influenced by serum levels of Vit D, & B12.
Know more about this report: Request for sample pages
Industry Dynamics
Growth Drivers
As eCOA meticulously collects data from both patients and the two doctors, hospitals & healthcare providers are data-enabled actions on the patient commitment to do research projects. The eCOA collects data on portable devices such as smartphones & tablets, chatbots, apps, & patients bring-your-own-device policies. It makes use of safe systems that adhere to administrative regulations for gathering clinical data.
The main growth drivers for the implementation of eCOA solutions in the anticipated time frame are the increased acceptance and popularity of mHealth devices as well as the promotion of trend-setting innovations for patient consideration. For instance, Melbourne Health Logistics' (MHL) Supplier Improvement Pilot Project, according to a May 2020 article. Ten small-to-medium-sized businesses (SMEs) from Australia are participating in a project that aims to use digitization to solve problems with inventory and supply chain management.
Report Segmentation
The market is primarily segmented based on delivery mode, end-use, and region.
|
By Delivery Mode |
By End-Use |
By Region |
|
|
|
Know more about this report: Request for sample pages
Web & cloud-based is predicted to dominate the industry's market segment.
Web and cloud-based technologies will dominate the market in 2022. The primary growth of the market is due to the benefits of its application. Some of the primary benefits provided by these systems include remote access & sharing to prevent potential data misuse.
Another advantage of this software is that it does away with the requirement for internal upkeep, which is likely the main factor driving their increased demand. Cloud-based systems are ideal for complex, multisite clinical investigations since everything is stored in a single location and is kept current by a third-party source. For instance, On July 2021, to provide healthcare organizations with the capacity to store, & query health in available cloud platforms.
Contract research organizations sector will dominate the market
Due to the outsourcing of clinical research management by important biopharmaceutical and medical device companies, the segment is predicted to increase significantly soon. Additionally, the expansion of the segment is facilitated by the development of new and inventive technologies that permit faster analysis and are more user-friendly. For instance, On April 2020, Pfizer Inc. and ICON plc announced a three-year partnership. The agreement expands on the businesses' already-existing partnership, which sees ICON supplying global experience in clinical trial strategy, execution, management, and conduct.
Rising demand for comprehensive health examinations carried out by clinical laboratories all over the world and the rising popularity of digital pathology portals also spur the segment growth.
The demand in North America is expected to witness significant growth
The creation of new drugs is anticipated to be fueled in the upcoming years by the presence of technologically cutting-edge research facilities, medical device producers, academic institutions, and hospitals, as well as better healthcare infrastructure will accelerate regional growth. Additionally, the use of eClinical platforms is expected to increase in the upcoming years as a result of initiatives and acquisitions made by important end users and market participants.
For instance, Signant Health introduced new updated its SmartSignals Randomization & Trial Supply Management (RTSM) solution on October 6, 2021. Modern interface designs, updated dashboards, improved reporting, and upgrades to self-service user administration are some examples of expanded features.
Similarly, Aural Analytics, announced in October 2020 that it has teamed with Signant Health to integrate its A2ETM application SDK & Web API across its “Health's Rater Station & TrialMax training, quality monitoring, & eCOA products. Through the lifespan and continuum of care, A2E aims to make cloud-based analytics and clinical-grade voice capture commonplace.
Competitive Insight
Some of the major players operating in the global market include IBM, IQVIA, Medidata Solutions, Clario, ArisGlobal, Signant Health, Oracle Corporation, Paraxel International Corporation, TransPerfect, CRF Health, Cloudbyz, Clime do Health GmbH, ClinCapture and OmniComm Systems.
Recent Developments
- On June 29, 2022, Signant Health, announced the most recent developments in its electronic Clinical Outcomes Assessment (eCOA) platform, Signant SmartSignals eCOA. The enhancements meet the needs of trial sponsors and CROs that require eCOA solutions that are highly customizable, simple to adopt and operate with any type of device.
- In March 2022, Amazon Web Services & THREAD introduced “THREAD platform” driven by enterprise-scale automated and constructed artificial intelligence-driven technologies to speed up and enhance contemporary clinical trials.
Electronic Clinical Outcome Assessment Solutions Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2024 |
USD 2.04 billion |
|
Revenue forecast in 2032 |
USD 6.27 billion |
|
CAGR |
15.0% from 2024 - 2032 |
|
Base year |
2023 |
|
Historical data |
2019 – 2022 |
|
Forecast period |
2024 - 2032 |
|
Quantitative units |
Revenue in USD billion and CAGR from 2024 to 2032 |
|
Segments Covered |
By Delivery Mode, By End-Use, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America; Middle East & Africa |
|
Key Companies |
IBM, IQVIA, Medidata Solutions, Clario, ArisGlobal, Signant Health, Oracle Corporation, Paraxel International Corporation, TransPerfect, CRF Health, Cloudbyz, Clime do Health GmbH, ClinCapture, and OmniComm Systems. |
FAQ's
The electronic clinical outcome assessment solutions market report covering key segments are delivery mode, end-use, and region.
Electronic Clinical Outcome Assessment Solutions Market Size Worth $6.27 Billion By 2032 .
The global electronic clinical outcome assessment solutions market expected to grow at a CAGR of 15.0% during the forecast period.
North America is leading the global market.
Electronic Clinical Outcome Assessment Solutions Market key driving factors are Increased acceptance and popularity of mHealth devices.
