
NUT Midline Carcinoma Treatment Market Size, Share, Trends, Industry Analysis Report
By Treatment (Chemotherapy, Targeted Therapy, Immunotherapy, Radiation Therapy), By Route Of Administration, By End User, and By Region – Market Forecast, 2025–2034
- Published Date:Nov-2025
- Pages: 126
- Format: PDF
- Report ID: PM6520
- Base Year: 2024
- Historical Data: 2020-2023
Overview
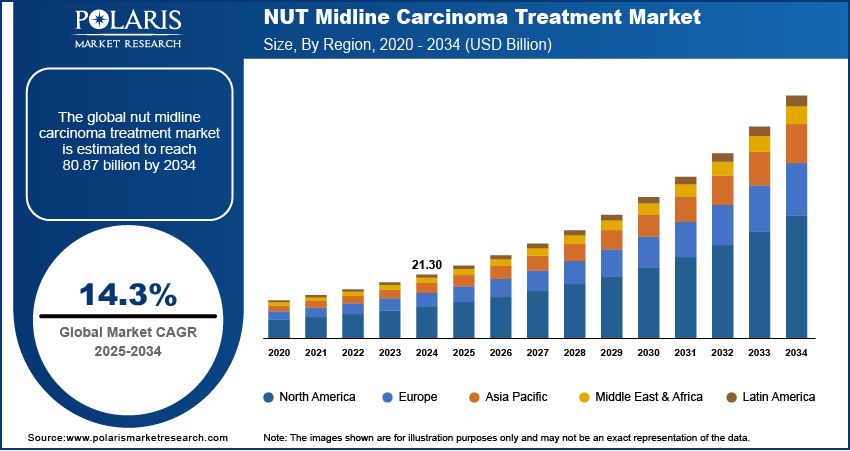
The global NUT midline carcinoma treatment market size was valued at USD 21.30 billion in 2024, growing at a CAGR of 14.3% from 2025 to 2034. Rising cancer incidence coupled with increasing healthcare expenditure is driving demand for NUT midline carcinoma treatment industry.
Key Insights
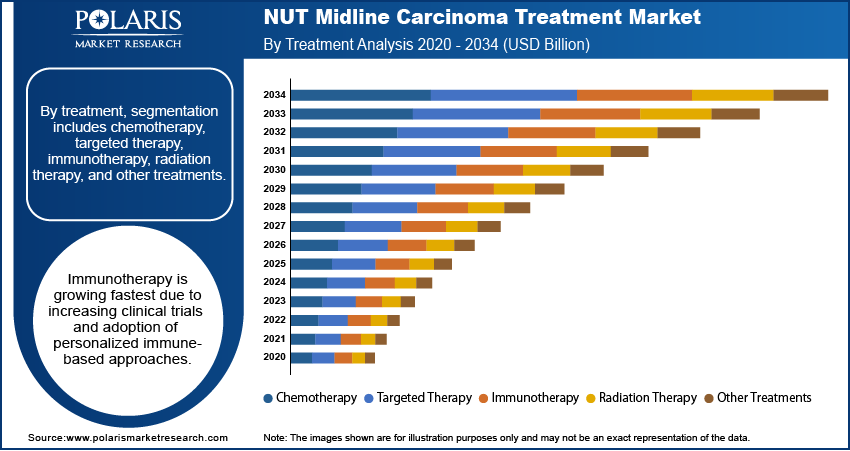
- Targeted therapy segment dominated the NUT midline carcinoma treatment market in 2024.
- Immunotherapy segment is projected to grow fastest due to increasing clinical trials and rising adoption of personalized immune-based treatments.
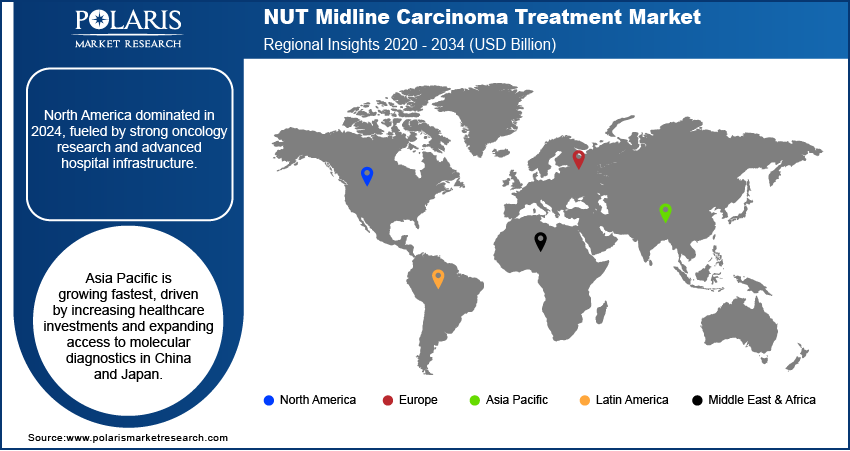
- North America held the largest share of the NUT midline carcinoma treatment market in 2024.
- The U.S. market is driven by advanced oncology infrastructure and strong precision medicine programs.
- Asia Pacific market is expected to grow from 2025 to 2034 due to expanding healthcare investments and growing molecular diagnostics adoption.
- China and Japan lead regional expansion through government support and increasing biopharma research capabilities.
Industry Dynamics
- Rising cancer incidence worldwide is increasing demand for advanced oncology diagnostics in NUT midline carcinoma treatment.
- Growing healthcare expenditure across major economies supports adoption of precision cancer therapies.
- Development of BET inhibitors and other novel targeted agents addressing NUTM1 fusion pathways while creating opportunity in this market.
- Delayed diagnosis and limited disease awareness hinders early adoption of treatment.
Market Statistics
- 2024 Market Size: USD 21.30 Billion
- 2034 Projected Market Size: USD 80.87 Billion
- CAGR (2025–2034): 14.3%
- North America: Largest Market Share
The NUT midline carcinoma treatment market is aimed at therapies and diagnosis for rare and aggressive tumors caused by NUTM1 gene fusions. The treatments encompass molecular diagnostics, targeted therapies, and research-focused methods that target enhancing patient outcomes and early diagnosis. The market is comprised of pharmaceutical firms, diagnostic companies, and research institutes to develop efficient therapeutic strategies for this rare disease.
Rising global cancer incidence and increasing healthcare expenditure are driving demand for advanced oncology diagnostics. According to the American Medical Association, U.S. health spending rose 7.5% in 2023 to USD 4.9 trillion (USD 14,570 per person), higher than the 4.6% increase in 2022 and second only to the 10.4% rise in 2020. Increasing emphasis on targeted therapy and personalized medicine is further driving research and clinical use for NUT midline carcinoma. Growing partnerships among biopharma firms and academic centers are fueling the identification of new treatment pathways.
However, lack of disease awareness, low patient volumes, and prohibitive diagnostic expense limit broad adoption. The lack of approved targeted therapies and standardized diagnostic protocols remains an ongoing obstacle. Despite these barriers, ongoing genomic investigation and orphan drug incentives hold large opportunities for future market expansion.
Drivers & Opportunities
Global increase in cancer incidence: The rising incidence of cancer worldwide is creating a greater need for early and accurate diagnosis, which is driving demand for advanced oncology diagnostics in the NUT midline carcinoma treatment market. New cases of cancer are projected to increase to more than 35 million by 2050, an increase of 77% from an estimated 20 million in 2022, says the WHO. With more patients diagnosed with aggressive and rare forms of cancers, health providers are increasingly adopting to molecular and rare disease genetic testing technologies to facilitate early intervention as well as personalized treatment approaches.
Rising healthcare expenditure: Growing healthcare expenditures within developed and developing economies is driving the adoption of advanced cancer diagnostics and targeted medicines. Higher budgets allow hospitals, research centers, and biotech companies to invest in precision medicine infrastructure, molecular testing, and innovative treatment approaches, thereby expanding access to NUT midline carcinoma diagnostics and therapies.
Segmental Insights
Treatment Analysis
By treatment, the market is divided into chemotherapy, targeted therapy, immunotherapy, and radiation therapy, other treatments. The targeted therapy segment led the market in 2024 due to its capability to selectively inhibit NUTM1 gene fusions, enhancing patient outcomes. Additionally, rising research and clinical acceptance of precision oncology continue to boost its high market position.
The immunotherapy segment is estimated to grow at the highest CAGR throughout the forecast period based on rising clinical trials and development of immune checkpoint inhibitors. In addition, rising adoption of personalized immuno-oncology approaches is accelerating market expansion.
Route Of Administration Analysis
Based on route of administration, the market is segmented into oral, intravenous (IV), and other. The intravenous administration segment led the market in 2024 due to its extensive application in delivering targeted and immunotherapy treatments effectively. Additionally, hospitals and specialized centers use IV routes in preference for accurate dosing and controlled therapy administration.
The oral administration market is poised to grow at the highest CAGR through the forecast period due to patient demand for convenient treatment options. Furthermore, the improvements in oral formulation of targeted therapies and small-molecule inhibitors are fueling adoption.
End User Analysis
Based on end user, the market is segmented into hospitals, specialty clinics, and other end users. Hospitals led the market in 2024 with advanced oncology facilities and access to multidisciplinary treatment teams. Additionally, hospitals enable early diagnosis and delivery of complex targeted and immunotherapy treatments.
The specialty clinics segment is expected to register the highest CAGR through the forecast period due to the growth of oncology-focused outpatient facilities. Furthermore, growing awareness and availability of precision cancer treatment in specialty clinics are driving adoption.
Regional Analysis
North America dominated the NUT midline carcinoma treatment market in 2024 owing to the high presence of advanced oncology facilities and high adoption of precision medicine. Furthermore, rising government and private investments in rare cancer research add to market growth. Additionally, the U.S. is home to multiple clinical trials on NUTM1-targeted therapies, which accelerate therapeutic advancement adoption.
The U.S. NUT Midline Carcinoma Treatment Market Insights
The U.S. dominated the North America market owing to extensive R&D in rare cancers and availability of advanced molecular diagnostic tools. In the U.S., a rare cancer affects around 15 people per 100,000 annually or has under 40,000 new cases each year, as stated by National Institutes of Health. Moreover, rising healthcare expenditure on precision oncology programs is driving early diagnosis and treatment adoption. In addition, favorable orphan drug incentives support therapeutic development.
Europe NUT Midline Carcinoma Treatment Market Assessments
Europe is expected to hold a significant share of the market by 2034 driven by established healthcare systems and increasing adoption of targeted therapies in oncology centers. Moreover, rising investments in rare cancer research and molecular diagnostics are driving market demand. In addition, countries such as Germany and the UK are implementing precision medicine initiatives to improve patient outcomes.
Asia Pacific NUT Midline Carcinoma Treatment Market Trends
Asia Pacific is projected to grow at the fastest CAGR during the forecast period due to increasing awareness of rare cancers and expanding healthcare infrastructure. The National Cancer Center of China defined rare tumors as those with an incidence of 2.5 per 100,000, based on the Chinese population characteristics. In addition, increasing government efforts supporting oncology research and early diagnosis initiatives are boosting market growth.
China NUT Midline Carcinoma Treatment Market Overview
China’s market growth is driven by expanding oncology hospitals and increasing access to molecular diagnostics. Furthermore, government initiative towards rare diseases promotes the usage of NUT midline carcinoma therapies. Moreover, partnerships with multinational pharmaceutical companies are accelerating clinical development.
Key Players & Competitive Analysis
The market for NUT midline carcinoma therapies is competitive with firms investing in the development of targeted therapies and increasing diagnostic capacity. Moreover, partnerships with multinational research institutions and pharmaceutical companies are driving clinical adoption and driving expansion in key markets.
Some of the large corporations in the treatment of NUT midline carcinoma include Merck & Co., Inc., Bristol-Myers Squibb Company, Pfizer Inc., F. Hoffmann-La Roche Ltd, C4 Therapeutics, Inc., Ipsen Biopharmaceuticals, Inc., GSK plc, Novartis AG, Syndax Pharmaceuticals Inc., ImageneBio Inc, Chimerix, NeoGenomics Laboratories, Zenith Epigenetics Ltd., and BioCentury Inc.
Key Players
- BioCentury Inc.
- Bristol-Myers Squibb Company
- C4 Therapeutics, Inc.
- Chimerix
- F. Hoffmann-La Roche Ltd
- GSK plc
- ImageneBio Inc
- Ipsen Biopharmaceuticals, Inc.
- Merck & Co., Inc.
- NeoGenomics Laboratories
- Novartis AG
- Pfizer Inc.
- Syndax Pharmaceuticals Inc.
- Zenith Epigenetics Ltd.
NUT Midline Carcinoma Treatment Industry Developments
July 2025: Zenith Epigenetics’ ZEN-3694, an oral BET inhibitor, received FDA Fast Track designation for treating metastatic or unresectable NUT midline carcinoma and is showing promising results in ongoing combination trials.
January 2025: Bristol Myers Squibb’s Trotabresib, a BET inhibitor in Phase II trials for solid tumors, shows a favorable development outlook based on historical oncology success rates.
NUT Midline Carcinoma Treatment Market Segmentation
By Treatment Outlook (Revenue, USD Billion, 2020–2034)
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Radiation Therapy
- Other Treatments
By Route Of Administration Outlook (Revenue, USD Billion, 2020–2034)
- Oral
- Intravenous (IV)
- Other
By End User Outlook (Revenue, USD Billion, 2020–2034)
- Hospitals
- Specialty Clinics
- Other End Users
By Regional Outlook (Revenue, USD Billion, 2020–2034)
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Netherlands
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Malaysia
- South Korea
- Indonesia
- Australia
- Vietnam
- Rest of Asia Pacific
- Middle East & Africa
- Saudi Arabia
- UAE
- Israel
- South Africa
- Rest of Middle East & Africa
- Latin America
- Mexico
- Brazil
- Argentina
- Rest of Latin America
NUT Midline Carcinoma Treatment Market Report Scope
|
Report Attributes |
Details |
|
Market Size in 2024 |
USD 21.30 Billion |
|
Market Size in 2025 |
USD 24.27 Billion |
|
Revenue Forecast by 2034 |
USD 80.87 Billion |
|
CAGR |
14.3% from 2025 to 2034 |
|
Base Year |
2024 |
|
Historical Data |
2020–2023 |
|
Forecast Period |
2025–2034 |
|
Quantitative Units |
Revenue in USD Billion and CAGR from 2025 to 2034 |
|
Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, and Industry Trends |
|
Segments Covered |
|
|
Regional Scope |
|
|
Competitive Landscape |
|
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, regions, and segmentation. |
FAQ's
The global market size was valued at USD 21.30 billion in 2024 and is projected to grow to USD 80.87 billion by 2034.
The global market is projected to register a CAGR of 14.3% during the forecast period.
North America led the market in 2024 due to advanced oncology infrastructure and widespread adoption of precision medicine technologies.
A few of the key players in the market are Merck & Co., Inc., Bristol-Myers Squibb Company, Pfizer Inc., F. Hoffmann-La Roche Ltd, C4 Therapeutics, Inc., Ipsen Biopharmaceuticals, Inc., GSK plc, Novartis AG, Syndax Pharmaceuticals Inc., ImageneBio Inc, Chimerix, NeoGenomics Laboratories, Zenith Epigenetics Ltd., and BioCentury Inc.
Targeted therapy dominated in 2024, driven by its ability to specifically inhibit NUTM1 gene fusions and improve patient outcomes.
Specialty clinics is growing steadily due to expansion of oncology-focused outpatient centers and rising adoption of personalized cancer therapies.
