
Asia Pacific Gaucher Disease Treatment Market Size, Share, Trends, & Industry Analysis Report
: By Type (Type 1, Type 3, Type 2), By Therapy, By Distribution Channel, and By Country – Market Forecast, 2025–2034
- Published Date:Jun-2025
- Pages: 129
- Format: PDF
- Report ID: PM5756
- Base Year: 2024
- Historical Data: 2020-2023
Market Overview
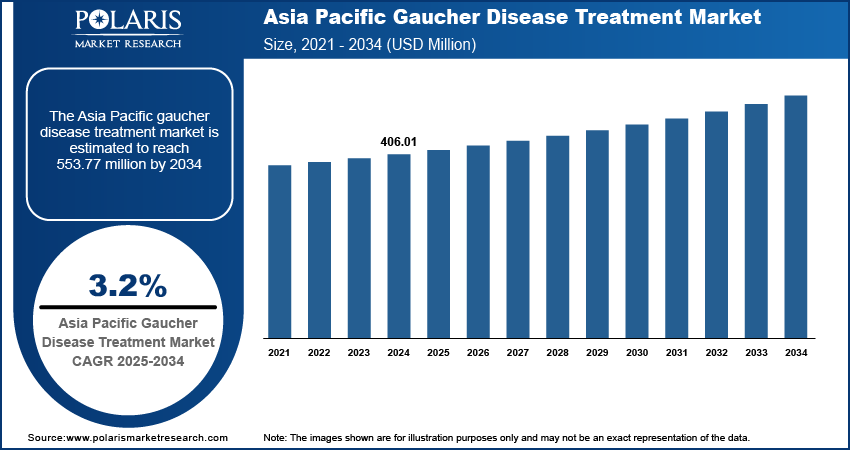
The Asia Pacific Gaucher disease treatment market was valued at USD 406.01 million in 2024 and is expected to register a CAGR of 3.2% during the forecast period. The growth is driven by rising healthcare spending and increasing strategic collaboration, acquisition, and partnership.
Gaucher disease is a rare genetic disorder caused by a deficiency of the enzyme glucocerebrosidase. This deficiency leads to the accumulation of glucocerebroside in cells, particularly in the spleen, liver, bone marrow, and occasionally the brain. As a result, individuals having Gaucher disease may experience symptoms such as enlarged organs, anemia, fatigue, bone pain, and, in severe cases, neurological complications. Treatment for Gaucher disease aims to manage these symptoms, replace the missing enzyme, or reduce the accumulation of the substrate. The primary treatment approaches include enzyme replacement therapy (ERT), substrate reduction therapy (SRT), and supportive care.
Asia Pacific is rapidly advancing in the Gaucher disease treatment landscape, supported by evolving healthcare systems, increased diagnostic awareness, and progressive policy frameworks. Historically, it has been underdiagnosed due to low disease awareness and limited access to advanced testing. Gaucher disease is gradually gaining more attention in the region, particularly in urban centers where specialized medical services are expanding. The increasing government focus on rare disease management is driving this shift toward treatment. For instance, in December 2024, the Indian High Court, Delhi, passed an order to create the National Fund for Rare Diseases (NFRD), allocating ~USD 117 million for 2024–2026, with similar or higher funding for 2026–2028. Several countries in the region are forming policy dialogues and strategic roadmaps aimed at improving access to rare disease diagnosis and treatment, which boosts investments in associated therapies and infrastructure, thereby driving the market growth.
Industry Dynamics
Expansion of Diagnostic and Genomic Testing Centers
Early detection of Gaucher disease is becoming more feasible as genomic technologies become more affordable and integrated into clinical workflows. Laboratories in Asia Pacific are increasingly equipped to handle enzyme assays and genetic sequencing, which are crucial for confirming Gaucher disease, thereby reducing diagnostic delays and supporting timely intervention. According to the January 2025 report by India's Ministry of Science & Technology, the FeED framework and IBDC portals are providing global researchers access to 10,000 whole genome samples from diverse Indian populations. Moreover, the region is witnessing rising collaboration between international pharmaceutical companies and local health institutions. In May 2023, the National Gaucher Foundation (NGF) collaborated with the Greenwood Genetic Center (GGC), a genetic diagnostics and research lab, to support NGF’s Global Diagnostic Initiative for improved genetic testing and clinical services. These partnerships are instrumental in facilitating knowledge transfer, establishing treatment protocols, and launching targeted educational campaigns for both clinicians and patients. This collaborative approach is also boosting the introduction of enzyme replacement therapies and substrate reduction therapies that are tailored to local healthcare delivery models, thereby driving the industry growth.
Rising Integration of Telemedicine in Region
The growing integration of telemedicine and digital health platforms is driving the market in Asia Pacific. According to a February 2025 report, India’s ABDM reported 739.8 million ABHA IDs created, 363,520 health facilities registered, 564,851 healthcare professionals onboarded, 159,020 facilities using ABDM software, and 490.6 million health records linked. Such initiatives are creating a digital health infrastructure for the country. They are expected to improve the continuation of care for patients affected by Gaucher disease in remote areas as these technologies mature, thereby driving the growth of the region.
Regional Volume and Pricing Analysis
|
Manufacturer |
Drug Name |
Dosage (IU/vial) |
Unit Price per Vial (USD) |
Annual Dose per Patient (Vials/Year) |
Patients (approx.) |
Total Sales Volume (Vials/Year) |
|
China |
Imiglucerase (Cerezyme), Velaglucerase alfa (VPRIV), Taliglucerase alfa (Elelyso) |
200 IU–400 IU |
1200–1,950 |
26–52 |
800–2,800 |
31,200–72,800 |
|
Japan |
xx |
xx |
xx |
xx |
xx |
xx |
|
India |
xx |
xx |
xx |
xx |
xx |
xx |
|
Taiwan |
xx |
xx |
xx |
xx |
xx |
xx |
|
South Korea |
xx |
xx |
xx |
xx |
xx |
xx |
|
Indonesia |
xx |
xx |
xx |
xx |
xx |
xx |
|
Malaysia |
xx |
xx |
xx |
xx |
xx |
xx |
|
Pakistan |
xx |
xx |
xx |
xx |
xx |
xx |
|
Rest of Asia Pacific |
xx |
xx |
xx |
xx |
xx |
xx |
Segmental Insights
By Type Analysis
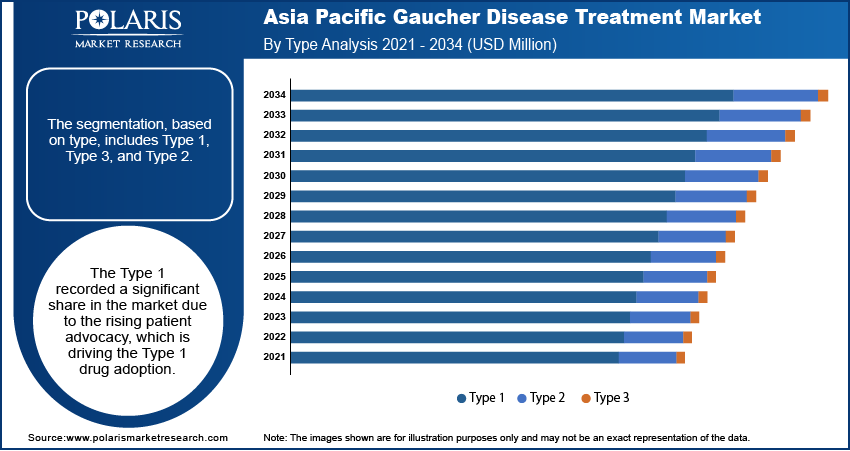
The segmentation, based on type, include type 1, type 3, and type 2. The type 1 segment dominated with the largest share and was values at USD 333.80 million in 2024. Type 1 Gaucher disease is the most common and mildest form. It mainly affects the liver, spleen, and bones, but does not involve the brain. As it is more widespread, it is more likely to be diagnosed and treated, especially with increasing awareness and improved screening. Patients often require long-term therapy, creating steady demand for treatments. The number of patients receiving treatment is growing as more people get tested and diagnosed early, thereby driving the segment growth.
The type 3 segment is projected to reach USD 92.21 million by 2034. Type 3 Gaucher disease is a chronic neuronopathic form that affects both the body and the brain. It is rarer than Type 1 but more serious, requiring complex and continuous medical care. Symptoms include neurological problems such as seizures, along with liver and spleen enlargement. The treatment approach for Type 3 is more challenging because most available therapies do not effectively treat brain-related symptoms. However, ongoing research clinical trials and virtual clinical trials are focusing on advanced therapies to address these issues, thereby driving the segment growth.
By Therapy Analysis
The segmentation, based on therapy, includes enzyme replacement therapy (ERT), substrate reduction therapy (SRT), and others. The enzyme replacement therapy segment accounted for 73.55% share in 2024, as enzyme replacement therapy is the most widely used treatment for Gaucher disease. It works by replacing the missing or faulty enzyme in the patient’s body, helping to reduce symptoms such as organ enlargement and bone problems. ERT is especially effective for Type 1 and some cases of Type 3 Gaucher disease. Since it has been proven to improve patients’ quality of life, it remains the first-line treatment. Though it requires regular intravenous infusions, many patients respond well, which is boosting its usage in disease treatment, thereby driving the segment growth.
By Distribution Channel Analysis
The segmentation, based on distribution channel, includes hospital pharmacies, retail pharmacies, and online pharmacies. The hospital pharmacies segment accounted for 68.40% share in 2024, as hospital pharmacies are the main distribution channel for disease treatments. This is because the treatments, especially enzyme replacement therapy, require careful handling, professional administration, and close medical supervision. Hospital pharmacies ensure that the medications are stored and delivered correctly, reducing the risk of side effects or complications. Many Gaucher disease patients visit hospitals regularly for their infusions, making these pharmacies a convenient and trusted source. Additionally, hospitals have specialists who monitor the patient’s progress and adjust treatment plans as needed. This makes hospital pharmacies essential in ensuring effective and safe treatment delivery for disease, which is driving the segment growth.
Country Analysis
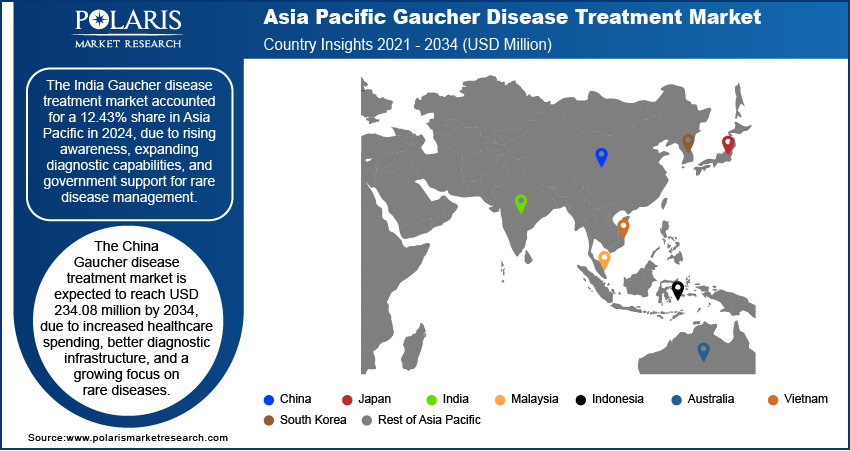
Gaucher Disease Treatment Market in India
The India Gaucher disease treatment market accounted for 12.43% revenue share in 2024 due to rising awareness, expanding diagnostic capabilities, and government support for rare disease diagnostics. Increased availability of genetic testing at lower costs is helping in early diagnosis. The launch of national rare disease policies and the inclusion of certain treatments under government health schemes are further driving the growth. Additionally, a growing number of specialty hospitals and improved healthcare access in urban areas are encouraging more patients to get diagnosis and treatment. Support from nonprofit organizations and growing interest from global pharma companies are further fueling the industry growth in India.
Gaucher Disease Treatment Market in China
The China Gaucher disease treatment market is expected to reach USD 234.08 million by 2034 due to increased healthcare spending, better diagnostic infrastructure, and a growing focus on rare diseases. Government programs aimed at improving rare disease awareness and access to treatment are helping more patients receive proper care. Rapid urbanization and a rising middle class with better access to healthcare services are driving the growth. Moreover, domestic biotech companies are beginning to invest in rare disease therapies, reducing dependency on imported drugs. Collaborations between Chinese hospitals and international pharmaceutical companies are driving the availability and adoption of advanced Gaucher treatments, thereby driving the industry growth.
Gaucher Disease Treatment Market in Taiwan
The Taiwan Gaucher disease treatment market accounted for a 2.88% share in 2024, driven by strong government healthcare programs and widespread access to genetic screening. The National Health Insurance system plays a vital role in covering treatment costs, which makes enzyme replacement therapy more accessible to patients. Early diagnosis through newborn screening initiatives further driver the industry. Additionally, Taiwan’s research institutions are actively involved in rare disease studies, which helps promote awareness and innovation. Partnerships with global pharmaceutical companies and a supportive regulatory environment have made advanced therapies more available, thereby driving the country growth.
Gaucher Disease Treatment Market in Japan
The Japan Gaucher disease treatment market accounted for a 21.09% share in 2024, driven by a well-established healthcare system, strong regulatory support, and a focus on precision medicine. The government actively supports rare disease research and provides reimbursement for high-cost therapies, including enzyme replacement therapy. Japan’s early adoption of advanced genetic testing has improved diagnosis rates, even for rare conditions such as Gaucher disease. Pharmaceutical companies benefit from orphan drug designations, which offer market exclusivity and financial incentives. Public awareness campaigns and support from patient advocacy groups also contribute to increased treatment uptake, thereby driving the growth.
Key Players and Competitive Analysis Report
Companies focus on strategic licensing, collaborations, and regional expansion to secure market share and enhance their product pipelines. Several investigational molecules are aimed at addressing central nervous system manifestations, with a growing preference for small-molecule oral agents that modulate glycosphingolipid biosynthesis. Research and development efforts are primarily directed toward therapies that lessen the need for frequent infusions and improve accessibility in underserved areas. Market participants actively pursue regulatory designations such as orphan drug status to accelerate the development process and gain market exclusivity. The development of biosimilars introduces additional competition, particularly in regions with cost-sensitive healthcare systems. As the understanding of Gaucher disease pathology advances, therapeutic approaches increasingly incorporate biomarker-driven strategies and personalized care. This dynamic environment fosters continuous innovation and intensifies competition across all segments of the Gaucher disease treatment market.
Key Players
- Amicus Therapeutics, Inc.
- CANbridge Life Sciences Ltd.
- Eli Lilly and Company
- Johnson & Johnson, Inc.
- Lingyi Biotechnology
- Protalix Biotherapeutics Inc.
- Sanofi
- Spur Therapeutics
- Takeda Pharmaceutical
Industry Developments
May 2025: CANbridge Pharmaceuticals successfully launched Gaurunning, China’s first domestically developed enzyme replacement therapy for Gaucher disease, following NMPA approval. The therapy demonstrated strong clinical results and marked a major advancement in local rare disease treatment accessibility and innovation.
Asia Pacific Gaucher Disease Treatment Market Segmentation
By Type Outlook (Revenue, USD Million, 2020–2034)
- Type 1
- Type 3
- Type 2
By Therapy Outlook (Revenue, USD Million, 2020–2034)
- Enzyme Replacement Therapy (ERT)
- Substrate Reduction Therapy (SRT)
- Others
By Distribution Channel Outlook (Revenue, USD Million, 2020–2034)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
By Country Outlook (Revenue, USD Million, 2020–2034)
- China
- Japan
- India
- South Korea
- Taiwan
- Indonesia
- Malaysia
- Pakistan
- Rest of Asia Pacific
Asia Pacific Gaucher Disease Treatment Market Report Scope
|
Report Attributes |
Details |
|
Market Size in 2024 |
USD 406.01 Million |
|
Market Size in 2025 |
USD 416.72 Million |
|
Revenue Forecast by 2034 |
USD 553.77 Million |
|
CAGR |
3.2% from 2025 to 2034 |
|
Base Year |
2024 |
|
Historical Data |
2020–2023 |
|
Forecast Period |
2025–2034 |
|
Quantitative Units |
Revenue in USD Million and CAGR from 2025 to 2034 |
|
Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, and Industry Trends |
|
Segments Covered |
|
|
Regional Scope |
|
|
Competitive Landscape |
|
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, regions, and segmentation. |
FAQ's
The market size was valued at USD 406.01 million in 2024 and is projected to grow to USD 553.77 million by 2034.
The market is projected to register a CAGR of 3.2% during the forecast period.
China dominated the market share in 2024.
A few of the key players in the market are Amicus Therapeutics, Inc.; CANbridge Life Sciences Ltd.; Eli Lilly and Company; Johnson & Johnson, Inc.; Lingyi Biotechnology; Protalix Biotherapeutics Inc.; Sanofi; Spur Therapeutics; and Takeda Pharmaceutical.
The type 1 segment dominated the market share in 2024.
The substrate reduction therapy segment is expected to witness the fastest growth during the forecast period.
