
C-Reactive Protein Testing Market Share, Size, Trends & Industry Analysis Report
By Assay Type (Immuno-turbidimetric Assay, ELISA, Chemiluminescence Immunoassay, Others); By Detection Range; By Disease Area; By End-Use; By Region; Segment Forecast, 2025 - 2034
- Published Date:Sep-2025
- Pages: 118
- Format: PDF
- Report ID: PM1770
- Base Year: 2024
- Historical Data: 2020-2023
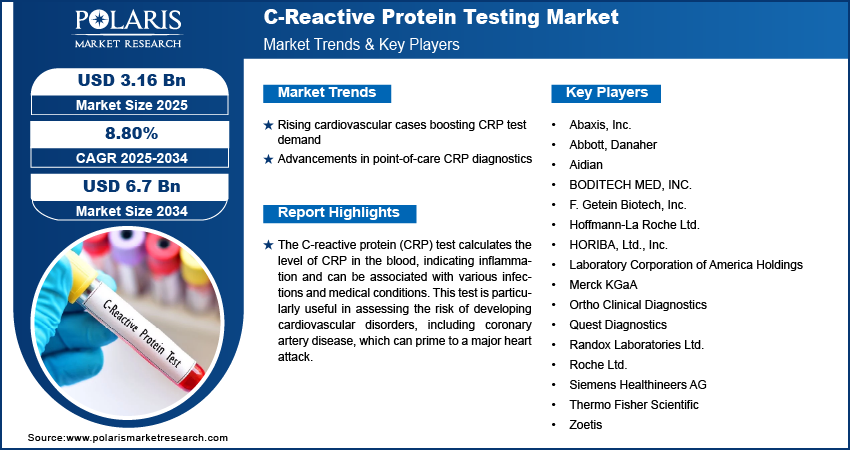
The global C-reactive protein (CRP) testing market was valued at USD 2.9 billion in 2024 and is projected to grow at a CAGR of 8.80% from 2025 to 2034. Market expansion is fueled by increasing incidence of inflammatory diseases and chronic conditions.
Key Insights
- The immunoturbidimetric assays segment accounted for the largest revenue share in 2024, due to the rapidly aging global population prone to immunological disorders.
- The high-sensitivity C-reactive protein (hs-CRP) testing segment is expected to witness the fastest market share during the forecast period, driven by advancements in CRP technologies.
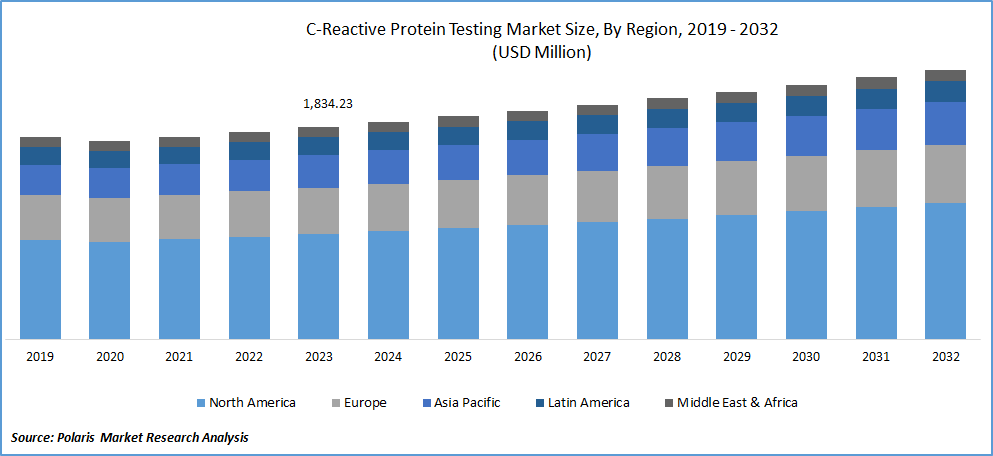
- In 2024, North America accounted for the largest market share, primarily driven by the high prevalence of cardiovascular diseases in countries like the U.S. and Canada.
- Asia Pacific is anticipated to grow at the fastest pace during the forecast period, fueled by the rising senior population in countries like Japan and China.
Industry Dynamics
- The increasing prevalence of cardiovascular diseases is projected to drive the demand for C-reactive protein (CRP) testing, as it provides information about inflammation, a major risk factor for cardiovascular disease (CVD).
- The rising aging population is fueling the adoption of C-reactive protein (CRP) testing, as elderly individuals have an increased risk of chronic inflammatory diseases and age-related conditions.
- The growing investment in healthcare spending is creating a lucrative market opportunity.
- The high cost and lacof standardized reimbursement are projected to hamper the market growth.
Market Statistics
- 2024 Market Size: USD 2.9 Billion
- 2034 Projected Market Size: USD 6.7 Billion
- CAGR (2025-2034): 8.80%
- North America: Largest Market Share
AI Impact on C-Reactive Protein Testing Market
- AI enables faster and more accurate CRP analysis by automating interpretation and reducing manual errors.
- Machine learning can detect inflammation-linked changes from ECG or large datasets.
- AI-driven data analytics improve clinical decision-making by cross-referencing CRP with patient history.
- Automation and AI integration streamline lab workflows, increase efficiency, and cut costs.
To Understand More About this Research: Request a Free Sample Report
The C-reactive protein (CRP) test calculates the level of CRP in the blood, indicating inflammation and can be associated with various infections and medical conditions. This test is particularly useful in assessing the risk of developing cardiovascular disorders, including coronary artery disease, which can prime to a major heart attack. The market demand for CRP testing is expected to increase due to the rising prevalence of cardiovascular diseases and the adoption of point-of-care devices.
Elevated levels of CRP in the plasma can indicate inflammation, which may be caused by a wide range of diseases, including inflammatory and malignant conditions. High readings of CRP testing can also indicate inflammation in the heart or blood vessels, thereby increasing the risk of heart disease.
Furthermore, the COVID-19 pandemic has positively impacted the growth of the CRP testing market. Due to increased demand for the test, the adoption of CRP testing has proven beneficial in the early detection and assessment of disease severity during COVID-19. This trend is expected to continue and drive market growth in the foreseeable future.
Plasmonic nanoparticles (PNPs) possess versatile optical properties, making them valuable tools for biosensing applications. A research study conducted by BabeTM-Bolyai University in Romania and published in July 2021 provided an overview of assays based on plasmonic nanoparticles for detecting C-reactive protein (CRP). The study also demonstrated using recently developed point-of-care assays for fast CRP testing. These advancements are expected to contribute to the accelerated growth of the C-reactive protein testing market.
C-reactive protein is considered a marker for evaluating the severity of infections and monitoring therapeutic progress, per the guidance provided by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). In addition, Chinese guidelines recommend using CRP tests, along with additional clinical parameters, for evaluating and following up on SARS-CoV-2 disorder. Organizations like Aidian and Abbott offer products that serve as supportive diagnostic tools in managing COVID-19 infections.
For example, Aidian provides user-friendly QuikRead go CRP tests and a portable QuikRead go instrument for quantitatively measuring CRP. Also, Abbott provides the Afinion CRP and Afinion 2 Analyzer test, which provides results in just 3 minutes. The increased demand for CRP testing in research and clinical settings, driven by the COVID-19 pandemic, is expected to impact market growth positively.
Industry Dynamics
- Growth Drivers
- Rising Prevalence of Cardiovascular Diseases
The increasing prevalence of cardiovascular diseases is projected to drive the industry's demand for c-reactive protein (CRP) testing. Detection of high levels of CRP is often associated with cardiovascular diseases. According to the World Health Organization (WHO), in 2019, around 17.9 million individuals expired from cardiovascular disorders, accounting for 32% of global deaths. Most (85%) of these fatalities were attributed to heart attacks or strokes.
Moreover, there is a growing focus on implementing point-of-care screening and innovative techniques for CRP testing, supported by government initiatives. These initiatives aim to enhance accessibility and convenience in diagnosing and monitoring cardiovascular diseases. The development of advanced techniques in CRP testing is expected further to drive the growth of the CRP testing market.
C-reactive protein (CRP) tests diagnose inflammatory disorders like endometriosis, inflammatory bowel disease, rheumatoid arthritis, cancer, lupus, and cardiovascular disease. Elevated CRP, along with risk factors like high blood pressure, hypertension, high cholesterol, and diabetes, indicates significant heart disease risk. Early identification reduces heart attack and stroke risks. CRP aids in discovering medicines for inflammatory diseases in vitro and in vivo, contributing to market growth.
Report Segmentation
The market is primarily segmented based on assay type, detection range, disease area, end-use, and region.
|
By Assay Type |
By Detection Range |
By Disease Area |
By End-Use |
By Region |
|
|
|
|
|
To Understand the Scope of this Report: Speak to Analyst
By Assay Type Analysis
- The immunoturbidimetric assays segment accounted for the largest revenue share in 2024
The immunoturbidimetric assays segment accounted for the largest revenue share in 2024. The application of this technique in chronic diseases of the respiratory and cardiac systems will contribute to its dominance. Advancements in the Chemiluminescence Immunoassay (CLIA) process, providing accurate results, will further drive growth in this segment.
Technological advancements in immunoturbidimetric assays will fuel market growth. Researchers worldwide are developing advanced and innovative assays to support rapid and precise CRP testing. For example, an examination publicized in January 2021 by investigators from D.M. Vasudevan Agappe Diagnostic Limited, India, established using a latex-enhanced immunoturbidimetric assay for highly sensitive and wide-range CRP detection in human serum.
By Detection Range Analysis
- The hs (high sensitivity) CRP segment expected to witness the fastest market share during the forecast period.
The high-sensitivity C-reactive protein (hs-CRP) testing segment expected to witness the fastest market share during the forecast period, driven by advancements in CRP technologies. Compared to traditional CRP tests, hs-CRP testing is highly sensitive and can detect even small increases in CRP levels in the human body. It makes it valuable in identifying the risk for coronary artery disease, contributing to the demand for this segment.
Moreover, the market is witnessing the development of various hs-CRP-based point-of-care (POC) testing devices by different market entities, further boosting the market growth. Technical improvements in conventional CRP methods are also expected to drive the development of the hs-CRP testing segment. Introducing nano-bio hybrid materials in CRP biosensors has appeared as an advantageous procedure for rapid and accurate CRP measurement using portable biosensors. In a study published in May 2021, researchers from Korea outlined the advances in spectroscopy-based, electrical CRP biosensors and electrochemical, comprised of nanomaterial hybrids and biomaterial. Such analyses are expected to boost the adoption of advanced CRP tools by end users, fostering the growth of the hs-CRP testing market.
North America accounted for the largest market share in 2024
In 2023, North America accounted for the largest market share, primarily driven by the high prevalence of cardiovascular diseases in countries like the U.S. and Canada. For instance, in 2019, the U.S. witnessed over 356,000 out-of-hospital cardiac arrests (OHCA) annually, with a significant fatality rate of over 90%. It, coupled with advanced healthcare facilities and government initiatives, is expected to propel the growth of the c-reactive protein testing market in the region.
Asia Pacific is anticipated to exhibit fastest growth during the forecast period, fueled by a continuously increasing population and a rising senior population in countries like Japan and China. The region is also experiencing healthy adoption of CRP testing in hospitals and a growing prevalence of malaria and cardiovascular diseases. These factors are anticipated to boost the market's growth in Asia Pacific in the coming years.
Key Market Players & Competitive Insight
The C-reactive protein (CRP) Testing Market is characterized by intense competition among key players striving to offer innovative solutions and maintain a significant market share. As a vital component in assessing inflammation and associated health risks, CRP testing has garnered attention from various healthcare organizations and diagnostic companies. This competitive landscape is shaped by factors such as technological advancements, product differentiation, strategic collaborations, and a focus on research and development.
Some of the companies working in the global CRP market include:
- Abaxis, Inc.
- Abbott, Danaher
- Aidian
- BODITECH MED, INC.
- F. Getein Biotech, Inc.
- Hoffmann-La Roche Ltd.
- HORIBA, Ltd., Inc.
- Laboratory Corporation of America Holdings
- Merck KGaA
- Ortho Clinical Diagnostics
- Quest Diagnostics
- Randox Laboratories Ltd.
- Roche Ltd.
- Siemens Healthineers AG
- Thermo Fisher Scientific
- Zoetis
Recent Developments
- In November 2022, LumiraDx launched its highly sensitive C-Reactive Protein (CRP) point-of-care antigen test across India. The test helps reduce unnecessary antibiotic prescriptions, combating antimicrobial resistance, and provides results in just four minutes using a small blood sample.
C-Reactive Protein Testing Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2025 |
USD 3.16 billion |
|
Revenue forecast in 2034 |
USD 6.7 billion |
|
CAGR |
8.80% from 2025 – 2034 |
|
Base year |
2024 |
|
Historical data |
2020 – 2023 |
|
Forecast period |
2025 – 2034 |
|
Quantitative units |
Revenue in USD billion and CAGR from 2025 to 2034 |
|
Segments Covered |
By Assay Type, By Detection Range, By Disease Area, By End-Use, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America; Middle East & Africa |
|
Customization |
Report customization as per your requirements with respect to countries, region and segmentation. |
FAQ's
C-Reactive Protein Testing Market Size Worth $6.7 Billion By 2034
The global key market players include Randox Laboratories Ltd., Laboratory Corporation of America Holdings, Quest Diagnostics, Aidian, Abaxis.
North America is contribute notably towards the C-reactive Protein Testing Market.
C-reactive Protein Testing Market is expected to grow at a CAGR of 8.80% during the forecast period.
C-reactive Protein Testing Market report covering key segments are assay type, detection range, disease area, end-use, and region.
