
Preclinical CRO Market Share, Size, Trends, Industry Analysis Report
By Service Type (Toxicology Testing, Bioanalysis & DMPK studies, Chemistry, Compound Management, Others); By Mode; By End-User; By Region; Segment Forecast, 2024 - 2032
- Published Date:Feb-2024
- Pages: 118
- Format: PDF
- Report ID: PM4513
- Base Year: 2023
- Historical Data: 2019-2022
Report Outlook
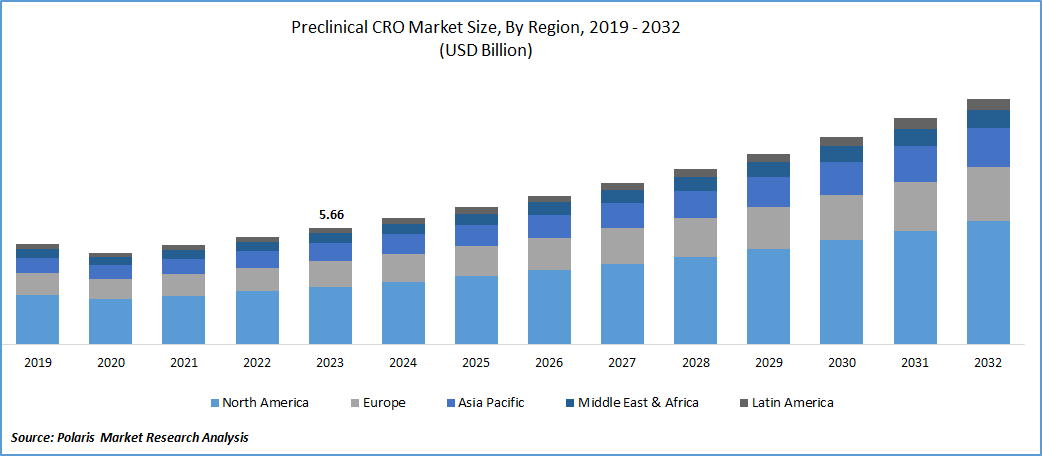
- Preclinical CRO Market size was valued at USD 5.66 billion in 2023.
- The market is anticipated to grow from USD 6.13 billion in 2024 to USD 11.90 billion by 2032, exhibiting the CAGR of 8.6% during the forecast period.
Market Introduction
The growing prevalence of chronic diseases and the need for novel therapies are fueling research and development activities in the pharmaceutical industry. This surge in R&D activities is translating into increased demand for preclinical CRO services. CROs that can provide a comprehensive range of preclinical services, including toxicology, pharmacokinetics, and bio analytical services, are well-positioned to capitalize on this opportunity.
Regulatory agencies are placing greater emphasis on safety and efficacy assessments during the drug development process. This has led to an increased demand for high-quality preclinical studies that adhere to rigorous regulatory standards. CROs with a strong track record of regulatory compliance and the ability to navigate complex regulatory landscapes are poised to thrive in this environment.
To Understand More About this Research: Request a Free Sample Report
- For instance, in November 2023, AmplifyBio, a prominent contract research organization, is unveiled the establishment of a new business division aimed at broadening its presence in the production of cutting-edge therapies, encompassing cell, gene, mRNA, and other advanced modalities.
One major trend in the preclinical CRO market is the increasing outsourcing of research activities by pharmaceutical and biotech companies. Outsourcing preclinical research to CROs allows these companies to tap into specialized expertise, reduce costs, and accelerate the drug development process. As a result, the demand for preclinical CRO services has risen, creating opportunities for CROs to expand their capabilities and cater to the evolving needs of their clients.
Advancements in technology is another significant trend shaping the preclinical CRO market. The integration of innovative technologies such as artificial intelligence, big data analytics, and high-throughput screening is enhancing the efficiency and accuracy of preclinical studies. This trend not only streamlines the drug discovery process but also allows CROs to differentiate themselves by offering cutting-edge, technology-driven solutions to their clients.
The research report offers a quantitative and qualitative analysis of the Preclinical CRO Market to enable effective decision-making. It covers the key trends and growth opportunities anticipated to have a favorable impact on the market. Besides, the study covers segment and regional revenue forecasts for market assessment.
Market Dynamics
Industry Growth Drivers
Increasing outsourcing trend is projected to spur the product demand.
The preclinical CRO market growth is greatly aided by an increasing trend of outsourcing the preclinical technologies. Pharmaceutical and biotechnology industries are increasingly outsourcing preclinical research activities to specialized Contract Research Organizations (CROs) to leverage their expertise, accelerate drug development, and manage costs effectively.
Growing investment in R&D is expected to drive preclinical CRO market growth.
Continued investment by pharmaceutical companies, exemplified by organizations like PhRMA, has contributed significantly to the growth of the preclinical CRO market, making the biopharmaceutical sector the most research and development-intensive industry.
Industry Challenges
Increasing competition among CROs is likely to impede the market preclinical CRO growth opportunities.
A notable obstacle arises from the intensifying competition among Contract Research Organizations (CROs), resulting in heightened pricing pressures and the potential for compromises in the delivery of service quality. The intricate and variable nature of preclinical study designs and endpoints presents another challenge, necessitating continuous adaptation by CROs to meet the diverse requirements of their clients. Moreover, the dynamic regulatory landscape poses a persistent challenge, requiring CROs to remain updated on evolving compliance standards and adeptly navigate regulatory complexities. Concerns related to data quality, reproducibility, and transparency in preclinical research also persist as critical challenges that demand meticulous attention and resolution within the industry.
Report Segmentation
The market is primarily segmented based on service type, mode, end-user, and region.
|
By Service Type |
By Mode |
By End-User |
By Region |
|
|
|
|
To Understand the Scope of this Report: Speak to Analyst
By Service Type Analysis
Bioanalysis & DMPK studies segment is expected to witness highest growth during forecast period
The bioanalysis & DMPK studies segment is projected to grow at a CAGR during the projected period in the preclinical CRO market. The growth of this sector is propelled by an increased need for pharmacokinetic services, particularly in supporting toxicology tests for IND-enabling studies. Bioanalysis and Drug Metabolism and Pharmacokinetics (DMPK) studies play a pivotal role across the entire drug development continuum, being integral in each stage rather than limited to the preclinical phase alone. This expansive application of bioanalysis and DMPK studies, along with the growing demand for these services, serves as a significant catalyst in driving the bioanalysis & DMPK studies growth.
By Mode Analysis
Patient-Derived Organoids (PDOs) segment is expected to dominate the preclinical CRO market during forecast period
In 2023, the preclinical CRO market share was predominantly influenced by Patient-Derived Organoids, commanding a significant market share. Patient-Derived Organoids (PDOs), derived from patient tissues and manifested as three-dimensional cell culture models, present a notably representative and personalized avenue for disease study and drug response testing. Their adeptness in replicating the intricacies of human tissues adds significant value to preclinical research. With the growing emphasis on precision medicine by pharmaceutical and biotech companies, there is a notable upsurge in the demand for PDO models. Contract Research Organizations (CROs) specializing in PDO models find themselves strategically positioned to seize this trend, offering clients advanced, patient-specific models that markedly improve the efficiency and efficacy of drug development processes.
By End-Users Analysis
Government and academic institutes segment is expected to witness highest growth during forecast period
The government and academic institutes segment is projected to experience the most rapid growth during the forecast period. Academic institutions and government entities play a pivotal role in the initial stages of discovery and development in the preclinical phase. The growing trend of academic organizations and government bodies outsourcing preclinical services to Contract Research Organizations (CROs) will significantly contribute to the expansion of this segment. Notably, academic institutes represent a substantial revenue source for major CROs like Charles River Laboratories and LabCorp, highlighting their pivotal role in the industry's revenue stream.
Regional Insights
North America region dominated the global preclinical CRO market in 2023
North America dominated the global preclinical CRO market in 2023 and is expected to continue to do so. The region's market growth is propelled by factors such as a rise in research and development (R&D) activities and an increasing demand for medications within the target population. The pharmaceutical sector in North America exhibits concentration, with companies increasingly outsourcing drug development activities to Contract Research Organizations (CROs). Furthermore, governmental investments in contract development and manufacturing organization (CDMO) capacities for drug substance and drug product manufacturing present opportunities for end users, fostering demand for preclinical CRO services in the region.
However, during the forecast period, Asia Pacific is poised to exhibit the most rapid growth. This acceleration is attributed to the evolving business model of multinational corporations (MNCs), where increasing R&D costs drive a surge in preclinical outsourcing within the Asia Pacific region. Countries like India and China, known for the cost-effectiveness of their Contract Research Organizations (CROs), play a pivotal role in this trend. Established companies from Western Europe and the U.S. strategically engage in analytical services, site research development, and clinical activities in the Asia Pacific, aiming to mitigate the expenses associated with research endeavors.
Key Market Players & Competitive Insights
The preclinical CRO market is fragmented and is anticipated to witness competition due to several players' presence. Major service providers in the market are constantly upgrading their technologies to stay ahead of the competition and to ensure efficiency, integrity, and safety. These players focus on partnership, product upgrades, and collaboration to gain a competitive edge over their peers and capture a significant market share.
Some of the major players operating in the global market include:
- Charles River Laboratories
- Covance (LabCorp)
- Envigo
- Eurofins Scientific
- ICON plc
- InVentiv Health
- Medpace
- MPI Research (Envigo)
- PAREXEL International Corporation
- Pharmaceutical Product Development, LLC (PPD)
- QuintilesIMS (IQVIA)
- Syngene International
- Toxikon Corporation
- WuXi AppTec
- Laboratory Corporation of America Holdings (LabCorp)
Recent Developments
- In December 2023, 10x Genomics, Inc. introduced the Xenium Catalyst Network. This global consortium comprises technically proficient research institutions aimed at expediting researchers' global access to Xenium proof-of-concept data. The network's four founding members will substantially enhance the capacity of 10x Genomics' Xenium Catalyst Program, an initiative launched earlier this year to provide technical access services for potential customers.
- In October 2023, OBiO Technology (Shanghai) Corp., Ltd. a globally renowned contract development and manufacturing organization specializing in cell and gene therapy, officially disclosed a collaboration agreement with Refreshgene Therapeutics (Refreshgene).
Report Coverage
The preclinical CRO market report emphasizes on key regions across the globe to provide better understanding of the product to the users. Also, the report provides market insights into recent developments, trends and analyzes the technologies that are gaining traction around the globe. Furthermore, the report covers in-depth qualitative analysis pertaining to various paradigm shifts associated with the transformation of these solutions.
The report provides detailed analysis of the market while focusing on various key aspects such as competitive analysis, service type, mode, end-users, and their futuristic growth opportunities.
Preclinical CRO Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2024 |
USD 6.13 billion |
|
Revenue forecast in 2032 |
USD 11.90 billion |
|
CAGR |
8.6% from 2024 – 2032 |
|
Base year |
2023 |
|
Historical data |
2019 – 2022 |
|
Forecast period |
2024 – 2032 |
|
Quantitative units |
Revenue in USD billion and CAGR from 2024 to 2032 |
|
Segments covered |
|
|
Regional scope |
|
|
Competitive Landscape |
|
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, region and segmentation. |
Report Customization
We provide our clients the option to personalize the Preclinical CRO Market report to suit their needs. By customizing the report, you can get data as per your format and definition. Also, the customization option allows you to gain a deeper dive into a specific segment, region, customer, or market competitor.
Browse Our Top Selling Reports
U.S. Garden Planter Market Size, Share 2024 Research Report
Alexipharmic Drugs Market Size, Share 2024 Research Report
Cleaning and Hygiene Products Market Size, Share 2024 Research Report
FAQ's
The global preclinical CRO market size is expected to reach USD 11.90 billion by 2032
Charles River Laboratories, Covance (LabCorp), Envigo, Eurofins Scientific, Pharmaceutical Product Development, LLC (PPD), are the top market players in the market
North America region contribute notably towards the global Point of Preclinical CRO Market
Preclinical CRO Market CAGR of 8.6% during the forecast period.
The Point of Preclinical CRO Market report covering key segments are service type, mode, end-user, and region.
