
Viral Clearance Market Share, Size, Trends, Industry Analysis Report
By Method (Viral Removal Method, Viral Inactivation Method, and Viral Detection Method); By Application; By End User; By Region; Segment Forecast, 2024 – 2032
- Published Date:Mar-2024
- Pages: 120
- Format: PDF
- Report ID: PM4445
- Base Year: 2023
- Historical Data: 2019-2022
Report Outlook
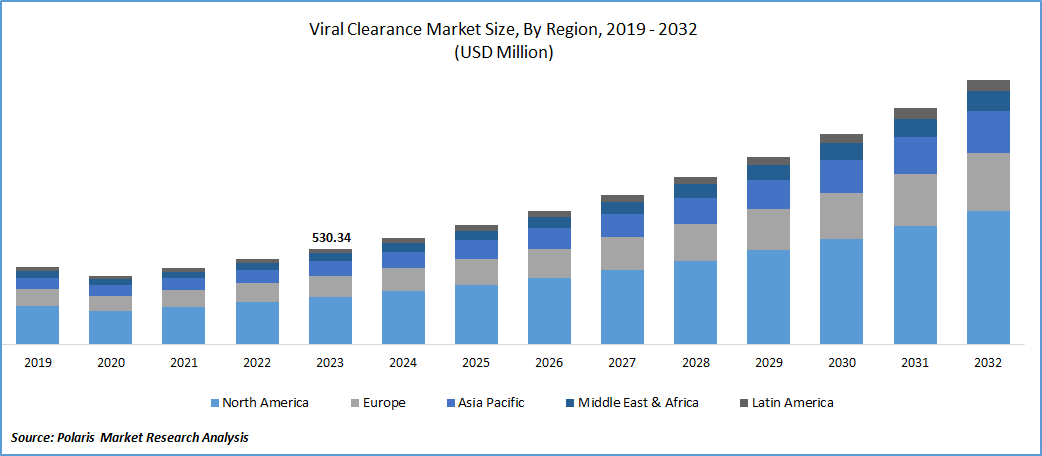
The global viral clearance market was valued at USD 530.34 million in 2023 and is expected to grow at a CAGR of 12.0% during the forecast period.
The constant emergence of viral clearance as a crucial process in the manufacturing of various types of biologics such as stem cell products biosimilar, biopharmaceuticals, driving the global market growth. The rise in number of new drug releases in different segments and demand for biopharmaceuticals for treating several chronic conditions, likely to bode well for global market’s growth. across the globe, are among the primary factors propelling global market growth.
In addition, the continuous expansion of pharmaceutical and biotechnologies industries and rising investments in research & development activities by both government and private organizations to introduce new products, are further anticipated to boost market’s growth.
To Understand More About this Research:Request a Free Sample Report
- For instance, in September 2022, MilliporeSigma, the U.S. and Canada Life Science business of Merck, announced about the opening of its new viral clearance lab of their new EUR 29 million Chia Biologics Testing Center. The newly developed center is first of its kind for the company in China and will allow customers to locally conduct studies.
The continuous increase in the advancements and improvements in nanofiltration technology that is widely used in viral clearance process for various purposes such as protein purification, downstream processing, better process control, and higher safety of biopharmaceutical products, will likely to create lucrative growth opportunities. For instance, in December 2023, TeraPore Technologies, announced the commercial launch of its flagship IsoBlock VF product line. The newly developed line of nanofiltration products leverage proprietary membrane technology.
However, the high costs associated with the various novel technologies of viral clearance and lack or scarcity of qualified or skilled specialists are key factors likely to restrain viral clearance market growth.
Industry Dynamics
Growth Drivers
- Increasing demand for biologics and vaccines and rise in R&D expenditure are major factors propelling market growth.
The rapid increase in the demand for several types of vaccines and biologics worldwide because of continuous growth in the number of people affecting from viral infections and different types of infectious diseases, is leading factor driving the market’s growth. For instance, according to the World Health Organization, there are over a billion cases of the seasonal influenza each year, and around 3-5 million cases of severe illness.
Additionally, with the growing investments in research & development activities by pharmaceutical and biotechnology companies for bringing new innovations and advancements in viral clearance processes and techniques, the market is growing at rapid pace and could generate new growth opportunities.
- For instance, in July 2023, Merck, announced that they have signed an agreement for the expansion of its reagent production in China. The company will invest USD 70 million to enable large-scale manufacturing of reagents for quality control & testing for the bio-pharma clients.

Report Segmentation
The market is primarily segmented based on method, application, end user, and region.
|
By Method |
By Application |
By End User |
By Region |
|
|
|
|
To Understand the Scope of this Report:Speak to Analyst
By Method Analysis
- Viral removal method segment accounted for the largest market share in 2023
The viral removal method segment accounted for the largest share. This dominance is accelerated by its numerous beneficial characteristics such as higher flexibility, efficiency, and cost-effectiveness, among others. Beside this, the growing proliferation and emergence of several new viral threats like novel viruses also lead to significant demand for effective viral clearance techniques like viral removal due to its ability to easily adapt to evolving types of viral.
The viral inactivation segment is projected to exhibit highest growth rate. This growth is due to rising demand for various new products including plasma proteins and gene therapy coupled with the growing adoption of the method due to its ability to provide higher patient safety by mitigating the risk of viral contamination.
By Application Analysis
- Vaccines segment held the majority market share in 2023
The vaccines segment held the majority market share. Segment’s dominance is attributable to increasing demand and proliferation for vaccines across the globe due to constant increase in the number of novel infections and growing incidences of various types of chronic diseases such as cervical cancer, hepatitis, and others.
- For instance, according to a report published by Center’s for Disease Control and Prevention, there are more than 296 million currently living with hepatitis B and around 58 million people living with hepatitis C. Also, it was estimated that 1.1 million deaths occurred due to these infections and their effects in 2019.
The blood and blood product segment is projected to register highest growth, on account of surge in demand for these products in various medical treatments, surgeries, and therapeutic interventions and growing awareness among health professionals about the potential benefits of such products.
By End User Analysis
- CROs segment is expected to witness highest growth
The CROs segment is expected will grow at highest pace. Segment’s growth is driven by substantial increase in the number of pharmaceutical and biotechnologies companies looking for outsourcing of early-phase development services and laboratory testing services because CROs often have specialized equipment, facilities, skilled personnel dedicated to viral clearance. Additionally, growing advancements in technology and analytical techniques have significantly improved the ability of CROs for conducting various viral clearance studies.
The pharmaceutical and biotech industry segment led the market. This growth is attributed to growing focus of companies towards bringing new innovations into the market and number of regulatory agencies promoting the development of new vaccines and drugs with improved characteristics to mitigate the risks associated with viral contamination.
Regional Insights
- North America region dominated the global market in 2023
The North America region dominated the global market. Region’s growth is driven by robust presence of leading biotechnology and medical institutes supporting the biotechnology and life science research and surge in the number of new drug approvals in the region. Additionally, the growing production of recombinant proteins & vaccines and awareness among the population regarding the use of biopharmaceuticals, is further escalating the market growth.
The Asia Pacific region is anticipated to emerge as fastest growing region. This growth is due to significant increase in the incidences of various chronic diseases and novel infections coupled with the exponential growth in medical research funding and growing number of CROs mainly in emerging economies. For instance, WHO, the number of people in India became diabetic between the period of 2019-2021 was estimated around 31 million and the prevalence of diabetes in India stands at 11.4%.
Key Market Players & Competitive Insights
The market is moderately competitive in nature with significant number of companies competing in the market to consolidate their position. Key players are competing on various factors such as improving the efficacy and safety of viral clearance, diversifying product portfolio, increasing regulatory compliance, and developing innovative viral clearance technologies, allowing them to gain a competitive edge in the market. For instance, in July 2023, MeMed, announced that they have received 510(k) clearance from the US FDA for the use of MeMed BV test on whole blood samples that will help healthcare providers distinguish between viral infections and bacterial in just 15 minutes.
Some of the major players operating in the global market include:
- Allure Medical Group
- Charles River Laboratories
- Clean Cells
- Creative Biogene
- Eurofins Scientific SE
- Kedrion
- Labor Dr. Merk & Kollegen GmbH
- Merck KGaA
- Microbiologics
- Sartorius AG
- Syngene International Limited
- Texcell
- ViruSure GmbH
- Wuxi Biologics
Recent Developments
- In December 2022, WuXi Biologics, announced about the launch of its new Biosafety Testing Center. The new 8,000 square-meter center will enhance the company’s capacity for cell bank characterization, virus harvest, unprocessed bulk harvest, and raw materials of animal origin release testing.
- In November 2022, Cygnus Technologies, a part of Maravai Life Sciences, introduced its new MockV RVLP Kit, that enables the bioprocess scientists to quantify removal of retrovirus-like particles.
Viral Clearance Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2024 |
USD 592.65 million |
|
Revenue forecast in 2032 |
USD 1464.76 million |
|
CAGR |
12.0% from 2024 – 2032 |
|
Base year |
2023 |
|
Historical data |
2019 – 2022 |
|
Forecast period |
2024 – 2032 |
|
Quantitative units |
Revenue in USD million and CAGR from 2024 to 2032 |
|
Segments covered |
By Method, By Application, By End User, By Region |
|
Regional scope |
North America, Europe, Asia Pacific, Latin America; Middle East & Africa |
|
Customization |
Report customization as per your requirements with respect to countries, region and segmentation. |
FAQ's
The key companies in Viral Clearance Market include Charles River Laboratories, Merck KgaA, Syngene International Limited, WuXi Biologics
The global viral clearance market is expected to grow at a CAGR of 12.0% during the forecast period.
Viral Clearance Market report covering key segments are method, application, end user, and region.
The key driving factors in Viral Clearance Market are Increasing demand for biologics and vaccines and rise in R&D expenditure are major factors propelling market growth.
Viral Clearance Market Size Worth $ 1464.76 Million By 2032.
