In-vitro Toxicology Testing Market Share, Size, Trends, Industry Analysis Report
By Product (Assays, Software, Consumables, Services, Instruments); By Method; By Technology; By End-Use; By Application; By Region; Segment Forecast, 2024- 2032
- Published Date:Feb-2024
- Pages: 118
- Format: PDF
- Report ID: PM4556
- Base Year: 2023
- Historical Data: 2019 – 2022
Report Outlook
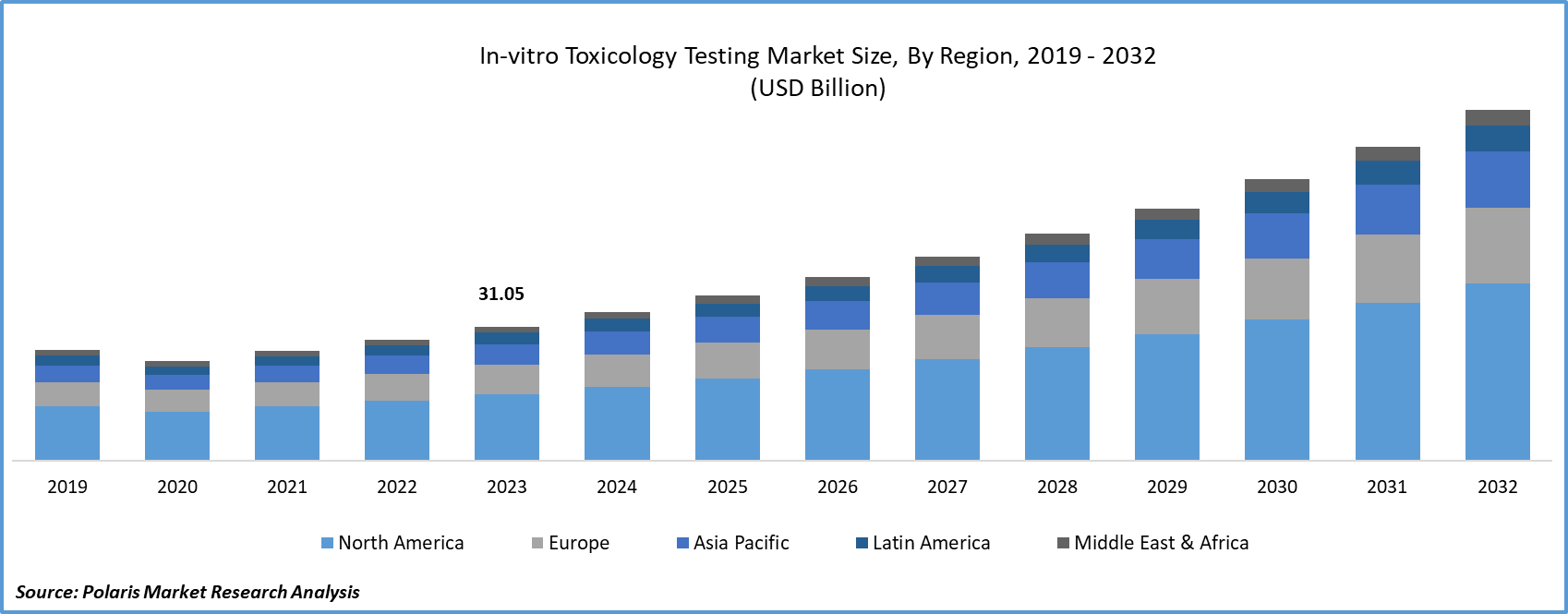
Global in-vitro toxicology testing market size was valued at USD 31.05 billion in 2023. The market is anticipated to grow from USD 34.41 billion in 2024 to USD 80.99 billion by 2032, exhibiting the CAGR of 11.3% during the forecast period
In-vitro Toxicology Testing Market Overview
Evaluating the potential toxicity of chemicals, drugs, and substances through non-animal testing methods is a crucial aspect of in vitro testing. This methodology utilizes cells, cellular components, or tissues outside their natural environment to gauge the safety and potential hazards of substances. The market for in-vitro tests encompasses a wide range of assays scrutinizing various toxicity facets, including genotoxicity, cytotoxicity, carcinogenicity, reproductive toxicity, organ toxicity, and environmental toxicity. These assessments are conducted on tissue models, cell cultures, or other in-vitro systems to simulate biological systems' responses to potential toxicants.
In the pharmaceutical industry, the primary focus is on quality management, ensuring that drugs are available as sterile, therapeutically active formulations that are reliable and consistent in performance. The continuous development of new medicinal agents at an accelerated pace is accompanied by the refinement of precise and sophisticated analytical methods for their assessment. Regulatory bodies like the Food and Drug Administration (FDA) provide industry guidance through various documents, covering topics such as safety testing of drug metabolites, in-vitro metabolism, transporter-mediated drug-drug interaction studies, and clinical drug interaction studies.
The pharmaceutical and biotechnology industry's growing demand for sterile medical products stems from the imperative of ensuring patient safety and protecting companies from potential product recalls. This heightened market demand underscores the increasing need for toxicology testing products in these industries.
To Understand More About this Research: Request a Free Sample Report
The pharmaceutical and medical device industries have witnessed transformative trends and breakthroughs, significantly enhancing global access to medicines. The impact of artificial intelligence and big data on disease diagnosis and treatment has been particularly noteworthy.
The success of pharmaceutical companies is closely linked to the effectiveness and safety of biopharmaceutical drugs, which not only have the potential to address previously incurable diseases but also contribute to industry prosperity. The existing biologics-development pipeline is supported by an opportunity for sustained healthy growth of market scope. Notably, the annual number of biotech patents applied since 1995 has seen a 25% increase. Currently, over 1,500 biomolecules are undergoing clinical trials, and the success rate of biologics has surpassed that of small-molecule drugs, with 13% of biopharmaceuticals entering phase I testing progressing to launch.
As these industries continue to thrive, the demand for toxicity testing to ensure the quality control of their products has risen proportionally. Thus, the rapid growth of the pharmaceutical and medical device industries acts as a major market trend for the expansion, and growth of the toxicity testing market.

In-vitro Toxicology Testing Market Dynamics
Market Drivers
Expanding pharmaceutical and medical device sectors bolstering the growth of the in-vitro toxicology testing market size.
The pharmaceutical and medical device sectors have experienced numerous groundbreaking trends and advancements, significantly enhancing the global availability of medicines for patients. The impact of artificial intelligence and big data on disease diagnosis and treatment has been evident.
Biopharmaceutical drugs, with their ability to address previously untreatable diseases, contribute to the success of pharmaceutical companies through their effectiveness and protection. A potential for sustained and healthy market growth supports the existing biologics development pipeline. Currently, over 1,500 biomolecules are undergoing clinical trials, with the success rate of biologics exceeding double that of small-molecule drugs. Notably, 13% of biopharmaceuticals entering phase I testing proceed to launch.
The prosperity and expansion of these industries directly drive the demand for toxicity testing to ensure quality control in their product development. Hence, the rapidly growing pharmaceutical and medical device industries market dynamics play a pivotal role in propelling the market share.
Market Restraints
The In-vitro Toxicology Testing and related products are burdened by a significant cost likely to hamper the growth of the market growth.
Automated toxicity testing systems, equipped with cutting-edge technology and advanced features, offer extensive functionalities. The automated AST ensures efficient and accurate toxicity detection. However, the market may be influenced by the high cost of these devices, encompassing advanced features such as e-tests, Microdilution, automated antimicrobial susceptibility testing (AST), rapid automated instrument methods, genotypic methods, disk diffusion method, MIC strip tests, and POCT-compatible technologies. By industry analysis and cost could pose a constraint on the In-vitro Toxicology Testing market size.
Report Segmentation
The market is primarily segmented based on product, method, technology, end-use, application, and region.
|
By Product |
By Method |
By End-Use |
By Application |
By Technology |
By Region |
|
Ex-vivo |
|
|
|
|
To Understand the Scope of this Report: Speak to Analyst
In-vitro Toxicology Testing Market Segmental Analysis
By Technology Analysis
- In 2023, the systemic toxicology segment took the lead in the market, primarily propelled by the increasing prevalence of research and development initiatives. Various private and public organizations are progressively endorsing the transition from animal testing to in-vitro techniques, with a specific focus on accurate systemic toxicity testing. Systemic toxicology testing provides a comprehensive outlook on the adverse effects of a substance across various physiological systems and organs. This holistic evaluation is crucial for gaining insights into the safety profile of the product. The favorable features of systemic toxicology testing are anticipated to create growth trend for this segment.
- Throughout the forecast period, the ocular toxicity segment is poised to experience a notable CAGR. Ocular toxicity arises from the intentional or unintentional exposure of ocular tissues to xenobiotics, which are foreign chemical substances capable of causing harm to the eyes. Additionally, the increasing awareness of ocular toxicity is expected to contribute to the expansion of this segment. For example, an article published by Oncology Nursing in April 2023 underscored the importance of comprehending ocular toxicity in the context of mirvetuximab soravtansine treatment.
By End-Use Analysis
- The pharmaceutical industry emerged as a dominant player in the market in 2023, holding a significant share. It stands as the primary market for in vitro toxicological testing, focusing on the identification of potential drug candidates at the early stages. The industry is actively implementing the principles of the 3 R's – replace, reduce, and refine – indicating its commitment to minimizing the use of animals in drug development processes and toxicological profiling.
- The Diagnostics segment is projected to achieve the fastest CAGR among other segments throughout the forecast period. This estimation is influenced by the presence of companies like Toxikon, specializing in tailored solutions to support the clinical development of diagnostic devices. Biocompatibility testing, a method used to assess the potential adverse effects of medical devices on human use, contributes to this anticipated market share. Additionally, companies offering extensive product lines for point-of-care drug testing, long-term alcohol abuse detection, and therapeutic drug monitoring are expected to enhance the growth of this segment.
In-vitro Toxicology Testing Market Regional Insights
The North America region dominated the global market with the largest market share in 2023
In recent years, government regulations support and advancing technology have facilitated the swift development of cost effective and innovative testing methods to ensure the safety of drugs, devices, chemicals, and cosmetics in the region. Substantial investments in tools and the expansion of laboratories in the region now empower clients to have comprehensive toxicological profiles for biopharmaceuticals, chemicals, cosmetics, and medical devices. These investments encompass high-throughput automation, multiplexing technologies, and screening, for biomarker analysis, the integration of flow cytometry and mass spectrometry facilities, and the expansion of existing cell/tissue culture capabilities.
Asia Pacific is expected to experience the fastest growth rate, driven by the growing focus of government-owned organizations on promoting toxicology testing through in-vitro toxicology methods. Additionally, clinical trials in the countries of Asia proves to be relatively cost-effective compared to nations in Western, leading some biopharmaceutical companies to conduct their drug development procedures in this region.
Competitive Landscape
The In-vitro Toxicology Testing market player is fragmented and is anticipated to witness competition due to several players' presence. Major service providers in the market are constantly upgrading their technologies to stay ahead of the competition and to ensure efficiency, integrity, and safety. These players focus on partnership, product upgrades, and collaboration to gain a competitive edge over their peers and capture a significant market share.
Some of the major players operating in the global market include:
- Agilent Technologies, Inc.
- Aragen Life Sciences Ltd.
- BioIVT
- Bio-Rad Laboratories, Inc.
- Catalent, Inc.
- Charles River Laboratories
- Creative Bioarray
- Creative Biolabs
- Enzo Biochem Inc.
- Eurofins Scientific
- Evotec SE
- Inotiv
- Intertek Group plc
- Laboratory Corporation of America Holdings
- Lonza
- MB Research Laboratories
- Merck KGaA
- Microbac Laboratories, Inc.
- Pacific BioLabs Inc.
- Promega Corporation
- Revvity
- SGS SA
- Shanghai Medicilon Inc.
- Thermo Fisher Scientific Inc.
- Vimta Labs Ltd.
Recent Developments
- In April 2023, Thermo Fisher Scientific introduced its inaugural CE-IVD certified real-time PCR assay kit designed for the testing of infectious diseases.
- In April 2023, Gentronix, Ltd extended its laboratory facilities to meet the increasing demand.
- In March 2023, Agilent Technologies, Inc. (US) completed the acquisition of e-MSion (US). This strategic move enables Agilent to incorporate e-MSion's ExD cell into its array of advanced workflows, instruments, and analytical solutions, particularly enhancing capabilities in biotherapeutic characterization and development.
- In February 2023, Evotec declared the transfer of Cyprotex US, LLC, a subsidiary of Evotec, from Watertown to Framingham, U.S. The motive behind this relocation is to facilitate the expansion of the new facility, aiming for quicker turnaround times.
Report Coverage
The In-vitro Toxicology Testing market report emphasizes on key regions across the globe to provide better understanding of the product to the users. Also, the report provides market insights into recent developments, trends and analyzes the technologies that are gaining traction around the globe. Furthermore, the report covers in-depth qualitative analysis pertaining to various paradigm shifts associated with the transformation of these solutions.
The report provides detailed analysis of the market growth while focusing on various key aspects such as competitive analysis, product, method, technology, end-use, application, and their futuristic growth opportunities.
In-vitro Toxicology Testing Market Report Scope
|
Report Attributes |
Details |
|
Market Size Value in 2024 |
USD 34.41 billion |
|
Revenue Forecast in 2032 |
USD 80.99 billion |
|
CAGR |
11.3% from 2024 – 2032 |
|
Base Year |
2023 |
|
Historical Data |
2019 – 2022 |
|
Forecast Period |
2024 – 2032 |
|
Quantitative Units |
Revenue in USD billion and CAGR from 2024 to 2032 |
|
Segments Covered |
By Product, By Method, By Technology, By End-use, By Application, By Region |
|
Regional Scope |
North America, Europe, Asia Pacific, Latin America; Middle East & Africa |
|
Customization |
Report customization as per your requirements with respect to countries, region and segmentation. |
FAQ's
key companies in In-vitro Toxicology Testing Market are Agilent Technologies, Inc., Aragen Life Sciences Ltd., BioIVT, Bio-Rad Laboratories, Inc., Catalent, Inc., Charles River Laboratories
In-vitro Toxicology Testing Market exhibiting the CAGR of 11.3% during the forecast period
The In-vitro Toxicology Testing Market report covering key segments are product, method, technology, end-use, application, and region.
key driving factors in In-vitro Toxicology Testing Market are • Expanding pharmaceutical and medical device sectors
In-vitro Toxicology Testing Market Size Worth $80.99 Billion By 2032