
Biological Safety Testing Product and Services Market Share, Size, Trends & Industry Analysis Report
By Product (Reagents & Kits, Instruments, Services); By Test Type; By Application; By Region; Segment Forecast, 2025 - 2034
- Published Date:Jun-2025
- Pages: 130
- Format: PDF
- Report ID: PM3097
- Base Year: 2024
- Historical Data: 2020-2023
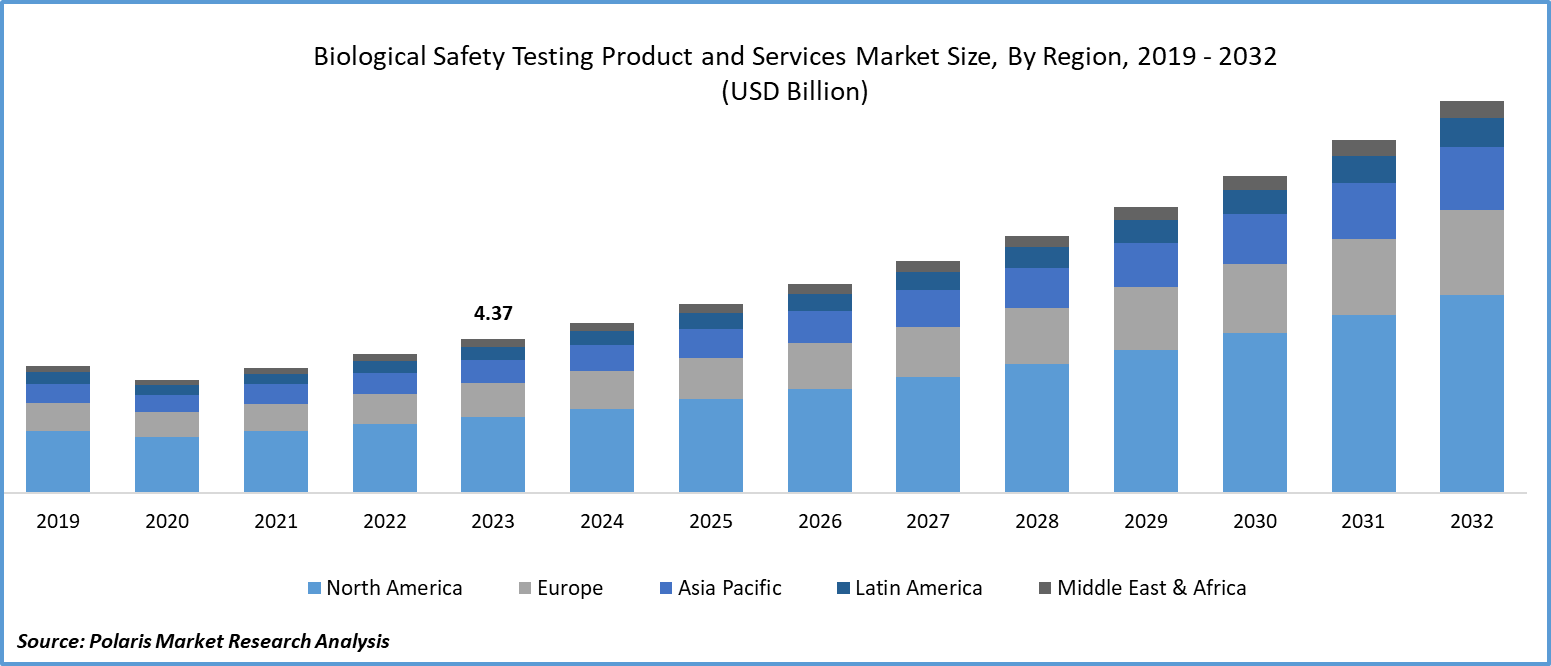
The global Biological Safety Testing Market was valued at USD 6.2 billion in 2024 and is projected to grow at a CAGR of 12.90% from 2025 to 2034. Stringent regulatory requirements for biopharmaceuticals and medical devices are fueling market expansion.
Market Overview
The biological safety testing products and services market is expected to witness significant growth in the study period owing to the increasing development of new therapeutics and treatments and the rising importance of evaluating their safety and effectiveness in treating health problems. The technological advancements in the healthcare industry are anticipated to positively influence the growth of the global market, according to the biological safety testing products and services market analysis.
For instance, in September 2023, HumanFirst introduced its new enterprise platform to promote artificial intelligence in clinical research.
Moreover, the rising interest in developing cell and gene therapies in the marketplace is widening the biological safety testing products and services market growth opportunities. The increased need to test the medicine before approval for common use in the marketplace is enabling companies to invest a significant amount in the testing of the biological safety of medicines. However, the higher costs of testing biological products and services are likely to hamper the growth of the market during the forecast period.
To Understand More About this Research: Request a Free Sample Report
Growth Drivers
Rising Investments in the Biopharmaceutical Space
The current biological safety testing products and services market trends are showcasing the enormous potential for market growth during the forecast period with the rising investments in biopharmaceutical production by the companies. For instance, in June 2023, Upstream Bio, a private healthcare investment company, raised $200 million in a Series B round that is likely to be used in the development of a monoclonal antibody for allergic and inflammatory diseases, UPB-101 in the next phase of clinical trials.
The Rising Government Initiatives to Promote Biopharmaceutical Safety
The COVID-19 pandemic created the necessity to innovate advanced vaccines and medicines researchers to stop the severity of spreading along with the increased need to evaluate the side effects of those medicines by government organizations with clinical trials. As an example, the U.S. Food and Drug Administration announced the establishment of the Coronavirus Treatment Acceleration Programme in 2020 to speed up the testing of therapeutics and drugs.

Restraining Factors
Regulatory Hurdles and Complex Testing Procedures
The need for advanced testing infrastructure for the testing of biopharmaceutical products, primarily cell and gene therapies, is expected to restrain the market growth of the biological safety testing products and services market. The lack of proficient employees with significant knowledge of the testing of biopharmaceutical products and services is hindering the growth of the biological safety testing products and services market.
Report Segmentation
The market is primarily segmented based on product, test type, application, and region.
|
By Product |
By Test Type |
By Application |
By Region |
|
|
|
|
To Understand the Scope of this Report: Speak to Analyst
By Product Analysis
Reagents & Kits Segment is Expected to Witness the Highest Market Growth During the Forecast Period
Based on the biological safety testing products and services market outlook, the reagents and kits segment is anticipated to grow at a higher CAGR during the forecast period due to the increasing demand for high-throughput testing. It is widely used in laboratory settings to monitor the presence of a range of substances in medicines. The rising concerns about drug safety and countering illegal drug use in the global space are showing a positive impact on the adoption of reagents and kits.
The instruments segment led the industry market with a substantial revenue share in 2023, largely attributable to the rising regulations in drug delivery. The utilization of various methods in biological drug testing is forcing companies to adopt compatible instruments, contributing to the significant demand for instruments in the marketplace.
By Test Type Analysis
Vaccines & Therapeutics Segment Registered the Largest Market Share in 2024
The vaccines and therapeutics segment witnessed the largest market share in 2023 and is likely to continue its growth trajectory throughout the study period. The growing demand for vaccines and therapeutics in the healthcare domain is propelling the need for proper infrastructure for biological testing in the coming years.
The gene therapy segment is expected to grow at the fastest rate over the next few years on account of rising concerns for product safety, quality, and lower side effects.
By Application Analysis
The Endotoxin Tests Segment Held a Significant Market Share in 2024
The endotoxin tests segment held a significant market share in 2023 due to the continuous rise in the manufacturing of biopharmaceutical products. Endotoxins are known to increase body temperature, which is why they are strictly avoided in the production of vaccines, therapies, and medicine to ensure the safety and efficacy of biopharmaceutical products. This is encouraging pharmaceutical companies to conduct endotoxin tests on their products.
The bioburden tests segment witnessed the second largest biological safety testing products and services market revenue share due to its profuse capability to monitor the presence of microbial contamination during the production of pharmaceutical products and services.
Regional Insights
North America Region Registered the Largest Share of the Global Market in 2024
The North American region dominated the global market. The growth of the segment market can be largely attributed to the presence of robust biopharmaceutical infrastructure and the stringent regulations on new therapeutics, which are the major factors contributing to the larger biological safety testing products and services market share in the global market.
The Asia Pacific region is expected to be the fastest-growing region with a healthy CAGR during the projected period, owing to the growing investments in the pharmaceutical industry in countries like India and China. For instance, Worg Pharmaceuticals, a Chinese company, raised $152 million in the June 2023 Series C round, which is used in developing immunotherapies related to autoimmune and allergic diseases.
Key Market Players & Competitive Insights
Strategic Acquisitions to Drive the Competition
The biological safety testing products and services market seems to be accompanied by a mix of consolidation and fragmentation, with the presence of certain larger players, along with growing mergers, acquisitions, and collaborative initiatives in the biopharmaceutical space to utilize collaborative power in the production and testing of therapies. For instance, in January 2024, AstraZeneca announced its plan to acquire Gracell, a cell therapy developer, for $1.2 billion.
Some of the major players operating in the global market include
- Agilent Technologies, Inc.
- Almac Group
- Associates of Cape Cod, Inc.
- BioMérieux SA
- Bio-Rad Laboratories, Inc.
- Charles River Laboratories, Inc.
- Eurofins Scientific
- F. Hoffmann-La Roche Ltd.
- FUJIFILM Wako Pure Chemical Corporation
- GenScript
- InvivoGen
- Labcorp
- Maravai Lifesciences
- Merck KGaA
- Microcoat Biotechnologie GmbH
Recent Developments in the Industry
- In October 2024, Merck opened a €290M biosafety testing facility in Rockville, USA, consolidating services into one site. Featuring Blazar CHO AOF, it delivers virus test results 60% faster to meet rising demand.
- In September 2023, Technical Safety Services, a portfolio company of Levine Leichtman Capital, entered a partnership with Controlled Environment Management, LLC, to enhance its testing capabilities in the pharmaceutical, biotechnology, and healthcare fields.
- In August 2023, the World Health Organization and the National Agency of Drug and Food Control, Indonesia, announced their plan to unveil new radioactive pharmaceutical product guidelines in early 2024.
Report Coverage
The biological safety testing products and services market report emphasizes key regions across the globe to provide a better understanding of the product to the users. Also, the report provides market insights into recent developments and trends and analyzes the technologies that are gaining traction around the globe. Furthermore, the report covers an in-depth qualitative analysis pertaining to various paradigm shifts associated with the transformation of these solutions.
The report provides a detailed analysis of the market while focusing on various key aspects such as competitive analysis, product, test type, application, and futuristic growth opportunities.
Biological Safety Testing Products and Services Market Report Scope
|
Report Attributes |
Details |
|
Market size value in 2025 |
USD 7 billion |
|
Revenue Forecast in 2034 |
USD 19.8 billion |
|
CAGR |
12.90% from 2025 – 2034 |
|
Base year |
2024 |
|
Historical data |
2020 – 2023 |
|
Forecast period |
2025 – 2034 |
|
Quantitative units |
Revenue in USD billion and CAGR from 2025 to 2034 |
|
Segments Covered |
|
|
Regional scope |
|
|
Competitive Landscape |
|
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, regions, and segmentation. |
Explore the 2024 market share, size, and revenue growth rate statistics in the field of Biological Safety Testing Products and Services Market, meticulously compiled by Polaris Market Research Industry Reports. This comprehensive analysis encompasses a market forecast outlook extending to 2032, along with an insightful historical overview. Experience the depth of this industry analysis by obtaining a complimentary PDF download of the sample report.
Browse Our Bestselling Reports:
G-Protein Coupled Receptors Market Size, Share Research Report
Conveying Equipment Market Size, Share Research Report
Chromium Market Size, Share Research Report
FAQ's
key companies in Biological Safety Testing Products and Services Market are Agilent Technologies, Inc., Almac Group, Associates of Cape Cod, Inc
Biological Safety Testing Products and Services Market exhibiting a CAGR of 12.90% during the forecast period.
The Biological Safety Testing Products and Services Market report covering key segments are product, test type, application, and region.
key driving factors in Biological Safety Testing Products and Services Market are Rising investments in the biopharmaceutical space
The global biological safety testing products and services market size is expected to reach USD 19.8 billion by 2034
