
Human Platelet Lysate Market Size, Share, Trends, & Industry Analysis Report
: By Application (Research and Therapeutic), By Type, By End Use, By Region – Market Forecast, 2025–2034
- Published Date:Jun-2025
- Pages: 113
- Format: PDF
- Report ID: PM3990
- Base Year: 2024
- Historical Data: 2020-2023
Market Overview
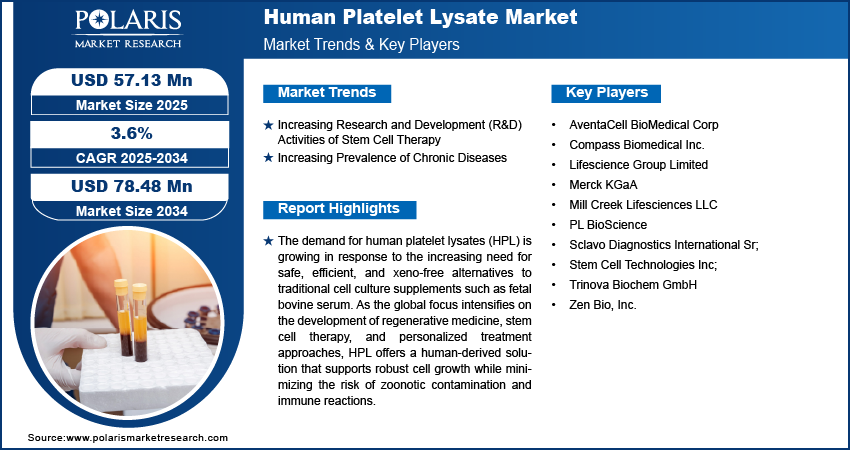
The global human platelet lysate market size was valued at USD 54.9 million in 2024, growing at a CAGR of 3.6% during 2025–2034.
Increasing concerns over the limitations and ethical issues associated with animal-derived products such as fetal bovine serum have accelerated the shift toward human-derived alternatives such as HPL. Additionally, the growing adoption of regenerative medicine, stem cell therapy, and advanced biologics is further expanding the demand for HPL.
Human Platelet Lysate (HPL) has emerged as a pivotal component in the field of biomedical research and regenerative medicine, revolutionizing cell culture techniques and therapeutic applications. This versatile product, derived from human blood platelets, offers numerous advantages over traditional cell culture supplements such as Fetal Bovine Serum (FBS). The growing demand for HPL can be attributed to several driving factors, including its safety, efficiency, and ethical considerations.
To Understand More About this Research: Request a Free Sample Report
HPL, in essence, is a growth supplement used in cell culture to promote the proliferation and maintenance of various cell types. Its unique composition, rich in growth factors and cytokines, makes it an ideal choice for researchers and clinicians alike. One of the primary driving factors behind the increasing use of HPL is its superior safety profile. Unlike FBS, HPL does not carry the risk of transmitting bovine diseases or causing immunological reactions, making it a safer option for both research and clinical applications. This enhanced safety aspect has garnered widespread recognition and trust within the scientific community.
Ethical considerations play a pivotal role in the increased demand for HPL. The use of FBS has long been criticized for its reliance on animal-derived products and the associated ethical concerns. HPL, being derived from human blood components, sidesteps these ethical issues and aligns more closely with the principles of responsible research. As the global scientific community becomes increasingly conscious of ethical considerations, HPL has emerged as a preferred alternative, reflecting a commitment to more humane and sustainable practices.
Market Dynamics
Increasing Research and Development (R&D) Activities of Stem Cell Therapy
The human platelet lysate market has witnessed a significant surge due to research and development (R&D) activities. Stem cell therapy, an emerging field with immense potential for regenerative medicine, has been a focal point of this growing interest. Governments around the world have initiated several strategic measures to support and advance R&D efforts in stem cell therapy. Governments have recognized the transformative potential of stem cell therapy in addressing a wide range of medical conditions, from degenerative diseases to injuries that were once considered irreversible. Consequently, they have allocated substantial funding and resources to foster innovation and facilitate clinical trials in this domain. By providing financial incentives, research grants, and streamlined regulatory pathways, governments have encouraged both public and private sector stakeholders to invest in the development of stem cell-based treatments.
Governments have played a crucial role in establishing ethical guidelines and frameworks for stem cell research and therapy. These regulations ensure that research activities are conducted with the highest standards of safety and ethics, which is particularly important given the sensitivity and complexity of stem cell-related work. These regulatory efforts have increased public trust in the field and paved the way for responsible advancements.
Government initiatives have facilitated international collaboration and knowledge sharing in stem cell research. Scientific exchange programs and partnerships with leading institutions have accelerated the pace of discovery and innovation. This global collaboration has had a cascading effect on the human platelet lysate market, as it is a vital component in many stem cell culture and expansion processes.
Increasing Prevalence of Chronic Diseases
The increasing prevalence of chronic diseases, including but not limited to Parkinson's disease, osteoarthritis, and androgenetic alopecia, has significantly impacted the human platelet lysate market. These chronic conditions have led to a growing demand for innovative and effective treatment options, driving research and development efforts in regenerative medicine.
Parkinson's disease, a progressive neurodegenerative disorder, has prompted extensive research into regenerative therapies that can potentially slow or reverse its debilitating effects. Platelet lysate, with its rich source of growth factors and cytokines, has become a valuable component in these therapies. Researchers are exploring the use of platelet lysate in cell-based treatments to stimulate neuronal regeneration and enhance the quality of life for individuals suffering from Parkinson's. As of July 25, 2022, data available from the National Institutes of Health (NIH) revealed that around 500,000 individuals are diagnosed with Parkinson's disease annually in the US.
Osteoarthritis, a common degenerative joint disease, affects millions worldwide and is a leading cause of pain and disability in the elderly. According to the World Health Organization (WHO), in 2019, about 528 million affected by osteoarthritis and about 73% of people suffering from osteoarthritis are older than 55 years. Regenerative approaches, including platelet lysate-based treatments, have gained attention for their potential to alleviate symptoms and restore joint function. Platelet lysate's ability to promote tissue repair and reduce inflammation makes it a promising candidate for use in regenerative therapies for osteoarthritis. Beyond these specific conditions, the rising prevalence of age-related health issues has created a demand for regenerative therapies that can address the root causes and provide long-term relief.
Segmental Insights
By Type Analysis
The heparin-free based HPL segment accounted for 41.2% market share in 2024, due to its advantages in regulatory compliance, safety, and broader applicability in clinical and research settings. Heparin-free Human Platelet Lysate has emerged as a critical component in cell culture and regenerative medicine, offering a serum-free alternative to traditional growth supplements. Captivate Bio launched PLTGold human platelet lysate that is a xeno-free, heparin-free supplement and is a superior alternative to FBS or human AB serum for the growth of MSCs and T cells. This innovative product is gaining traction in this sector that have reshaped the landscape of cell culture research and therapeutic development.
The global trend toward reducing the use of animal-derived components in cell culture has boosted the adoption of human platelet lysate. Heparin-free Human Platelet Lysate aligns with the principles of animal welfare and reduces the risk of transmitting zoonotic diseases, making it a sustainable and ethical choice for researchers worldwide. Another significant factor driving the market for heparin-free platelet lysate is its regulatory compliance. As regulatory agencies continue to tighten their guidelines for cell therapy and regenerative medicine, the need for well-defined and documented components in cell culture becomes paramount. Heparin-free Human Platelet Lysate satisfies these requirements and ensures product consistency, helping researchers navigate the complex regulatory landscape more confidently.
By Application Analysis
The research segment accounted for the largest share at 54.0% in 2024, driven by the growing use of human platelet lysate as a superior alternative to fetal bovine serum in cell culture research. It provides rich growth factors essential for expanding mesenchymal stem cells, immune cells, and other human-derived cells, making it ideal for preclinical and translational studies. Additionally, the rising number of academic and industrial research projects in regenerative medicine, cancer biology, and cell therapy drives demand. One of the primary applications of HPL in research is in cell culture. Traditional cell culture media often contain fetal bovine serum (FBS) as a supplement to provide essential nutrients and growth factors for cells. Human platelet lysate serves as a suitable alternative to FBS, providing a consistent and animal-free source of growth factors. Researchers are using hPL to culture a wide range of cell types, including stem cells, primary cells, and various cell lines. Its ability to support cell growth and maintain cell viability makes it an indispensable tool for in vitro studies.
The therapeutic segment is experiencing significant growth of 3.7% during the forecast period, due to the expanding use of cell-based therapies in treating chronic and degenerative diseases such as orthopedic disorders, cardiovascular conditions, and neurological disorders. This xeno-free profile significantly reduces the risk of immune reactions and transmission of zoonotic diseases, which is critical for clinical applications. The growing prevalence of conditions such as osteoarthritis, spinal cord injuries, and chronic wounds has driven demand for innovative therapies that promote tissue regeneration and healing areas where HPL plays a vital role.
By End Use Analysis
The pharmaceutical & biotechnological companies segment dominated the market, valued at USD 28.3 million in 2024. This dominance is attributed to their leading role in advancing cell-based therapies, biologics, and regenerative medicine. These companies are heavily invested in clinical research and large-scale cell manufacturing processes, where HPL is used as a critical supplement for expanding human cells under xeno-free and GMP-compliant conditions. Additionally, ongoing clinical trials and commercial production of advanced therapeutics require consistent and high-quality cell culture conditions, which HPL readily supports.
Collaborations and partnerships within the pharmaceutical and biotechnological industries have accelerated the growth of the human platelet lysate industry. These collaborations enable the development of standardized production processes, ensuring consistent quality and supply of human platelet lysate for research and therapeutic applications. On May 2022, AffyXell collaborated with GenScript to expedite the progress of stem cell-based therapies.
Regional Analysis
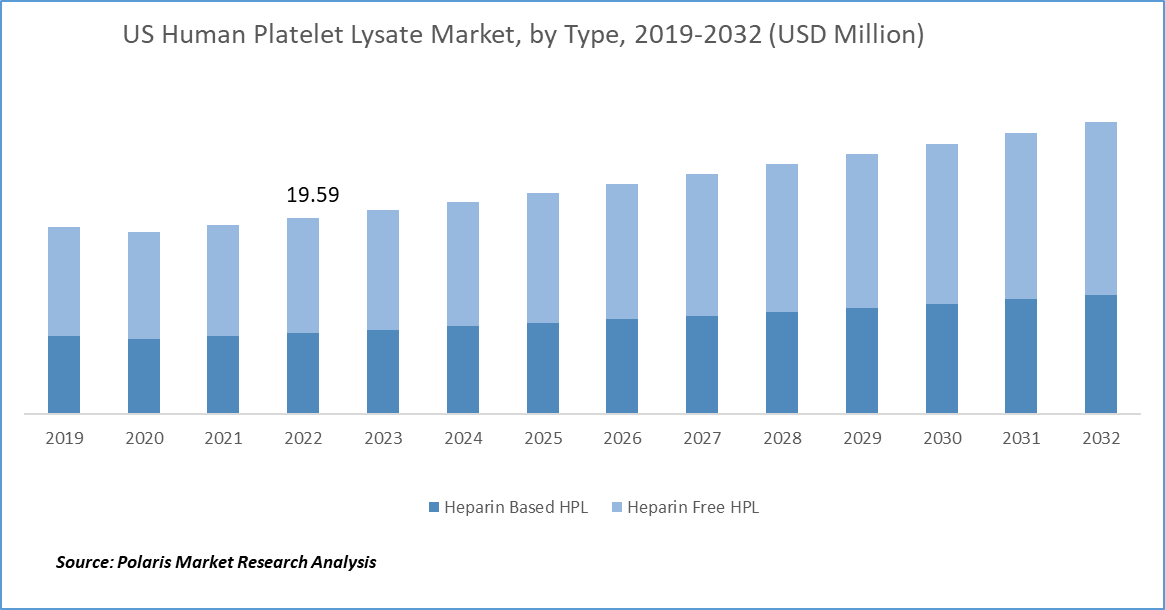
The North America human platelet lysate market size reached USD 23.93 million in 2024. North America is a hub for stem cell research, encompassing pluripotent stem cells, induced pluripotent stem cells (iPSCs), and adult stem cells. HPL is employed to culture and expand these cells for research into developmental processes and therapeutic applications. In the US, stem cell culture serves as a fundamental element in the field of regenerative medicine and biomedical research.
US Human Platelet Lysate Market Insight
The human platelet lysate market in US dominated in North America, capturing 88.7% regional market share, driven by several key factors. The US has a highly developed biotechnology and pharmaceutical industry, with significant investment in regenerative medicine, cell and gene therapy, and advanced biologics. This creates a strong demand for high-quality, xeno-free cell culture supplements such as HPL. The country also benefits from a robust healthcare infrastructure, a large number of academic and research institutions, and well-established biomanufacturing facilities, all of which support widespread adoption of HPL in both research and therapeutic applications.
Europe Human Platelet Lysate Market
The human platelet lysate market in Europe is projected to reach USD 19.28 million by 2034, owing to the region’s strong emphasis on biomedical research, expanding regenerative medicine sector, and favorable regulatory landscape. In Europe, ethical committees and regulatory bodies are responsible for supervising the utilization of HPL to guarantee that both research and clinical applications adhere to well-established ethical principles and do not jeopardize the well-being of donors or patients. Ethical oversight in Europe regarding human platelet lysate (HPL) involves rigorous monitoring and regulation to ensure that all research and clinical applications of HPL comply with established ethical standards. This oversight is designed to safeguard the welfare of both donors and patients. HPL applications are subject to ethical review, with a focus on responsible and safe use in biomedical and regenerative medicine research.
Government funding and collaborative initiatives in countries such as Germany, the UK, France, and the Netherlands have accelerated research in stem cell therapy, immunotherapy, and tissue engineering. The growing network of biopharmaceutical companies and academic research institutions in Europe is also contributing to the increased adoption of HPL in clinical and preclinical applications.
Asia Pacific Human Platelet Lysate Market Overview
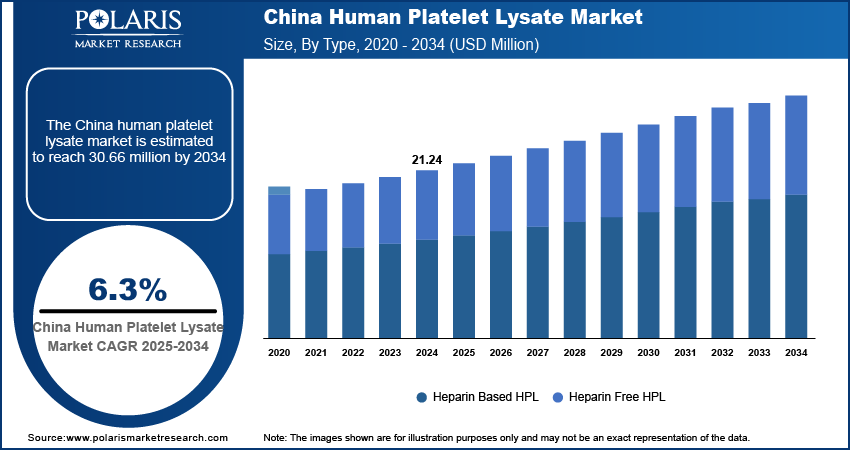
The human platelet lysate market in Asia Pacific accounted for 16.4% share of the global market in 2024 and is expected to register growth rate of 3.5% during the forecast period. Platelet lysate is a valuable component in cell culture and regenerative medicine, driving its demand in various research and clinical applications. The increasing prevalence of chronic diseases and the rising demand for regenerative therapies drive the market in the region. As the region grapples with a growing aging population and a higher incidence of degenerative diseases, there is a greater need for innovative treatments, such as stem cell therapies and tissue engineering, which often rely on human platelet lysate as a crucial component.
Asia Pacific has seen an expansion in the biotechnology and pharmaceutical industries, with many companies focusing on cell-based therapies and regenerative medicine. This has led to increased production and utilization of human platelet lysate in cell culture and research applications.

Key Players and Competitive Analysis
A few key players in the market are Merck KGaA; Compass Biomedical Inc.; PL BioScience; AventaCell BioMedical Corp; Mill Creek Lifesciences LLC; Stem Cell Technologies Inc;, Zen Bio, Inc.; Sclavo Diagnostics International Sr; Lifescience Group Limited; and Trinova Biochem GmbH. The market is highly competitive owing to the presence of various prominent players. Players have adopted strategies such as acquisition, launch, collaborations, and partnerships, and are engaging in the development of new products with high speed and improved features to enhance their product portfolio and hold a strong position in the market.
Key Players
- AventaCell BioMedical Corp
- Compass Biomedical Inc.
- Lifescience Group Limited
- Merck KGaA
- Mill Creek Lifesciences LLC
- PL BioScience
- Sclavo Diagnostics International Sr;
- Stem Cell Technologies Inc;
- Trinova Biochem GmbH
- Zen Bio, Inc.
Industry Developments
June 2023: PL BioScience GmbH entered into a Patent License and Assignment Agreement with French company Macopharma S.A.S., granting PL BioScience a worldwide license under Macopharma's patents and introducing PL BioScience to Macopharma's HPL customers for future orders.
March 2022: STEMCELL Technologies Inc. and PBS Biotech Inc. joined forces in a strategic supply partnership to offer the PBS-MINI Bioreactor through STEMCELL Technologies. This collaboration enables researchers aiming to expand their human pluripotent stem cell (hPSC) cultures to access this innovative bioreactor solution.
April 2022: BC Children’s Hospital Foundation introduced advanced stem cell research technology at BC Children’s Hospital ("BC Children’s"). This groundbreaking technology marks the first instance in history where all facets of stem cell culturing have been integrated and automated into a single piece of equipment.
Human Platelet Lysate Market Segmentation
By Type Outlook (Revenue, USD Million, 2020–2034)
- Heparin Based HPL
- Heparin Free HPL
By Application Outlook (Revenue, USD Million, 2020–2034)
- Research
- Therapeutic
By End Use Outlook (Revenue, USD Million, 2020–2034)
- Pharmaceutical & Biotechnological Companies
- Academic & Research Institutes
- Others
By Regional Outlook (Revenue, USD Million, 2020–2034)
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Netherlands
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Malaysia
- Australia
- Indonesia
- Rest of Asia Pacific
- Middle East & Africa
- Saudi Arabia
- UAE
- Israel
- Rest of Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
Human Platelet Lysate Market Report Scope
|
Report Attributes |
Details |
|
Market Size Value in 2024 |
USD 54.9 Million |
|
Market Size Value in 2025 |
USD 57.13 Million |
|
Revenue Forecast by 2034 |
USD 78.48 Million |
|
CAGR |
3.6% from 2025 to 2034 |
|
Base Year |
2024 |
|
Historical Data |
2020–2023 |
|
Forecast Period |
2025–2034 |
|
Quantitative Units |
Revenue in USD Million and CAGR from 2025 to 2034 |
|
Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, and Industry Trends |
|
Segments Covered |
|
|
Regional Scope |
|
|
Competitive Landscape |
|
|
Report Format |
|
|
Customization |
Report customization as per your requirements with respect to countries, regions, and segmentation. |
FAQ's
The global market size was valued at USD 54.9 million in 2024 and is projected to grow to USD 78.48 million by 2034.
The global market is expected to register a CAGR of 3.6% during the forecast period.
Asia Pacific held the largest share of the global market in 2024.
A few key players in the market are Merck KGaA; Compass Biomedical Inc.; PL BioScience; AventaCell BioMedical Corp; Mill Creek Lifesciences LLC; Stem Cell Technologies Inc; Zen Bio, Inc.; Sclavo Diagnostics International Sr;, Lifescience Group Limited; and Trinova Biochem GmbH.
The heparin-free based HPL segment accounted for 41.2% market share in 2024.
The pharmaceutical & biotechnological companies segment held the largest share of the global market in 2024.
